Biomed Res Bull. 2(2):79-86.
doi: 10.34172/biomedrb.2024.13
Review Article
Novel Electrochemical Biosensors for Detecting Specific Colorectal Cancer Biomarkers, Delving Into Advancements in Early Detection and Personalized Treatment Strategies
Zheng Dong 1  , Zheifeng Xu 1, *
, Zheifeng Xu 1, * 
Author information:
1Department of Immunology, Zhejiang University, Zhejiang, China
Abstract
The rising incidence and mortality rates of cancer have spurred advancements in precise and effective early diagnosis methods. This study aimed to explore electrochemical biosensors for detecting colorectal cancer (CRC). Biomarkers are crucial in screening tests and the treatment, prognosis, and medical management of CRC. Quick and accurate detection of these biomarkers aids in the early diagnosis of CRC. The article reviews various electrochemical recognition methods for CRC biomarkers, including nanomaterials and immunosensors. It also discusses the construction of electrochemical biosensors for distinguishing CRC-associated biomarkers. The combination of electrochemical biosensors offers high sensitivity, selectivity, and stability due to nanomaterials’ unique physical and chemical properties, easy surface functionalization, high electrochemical activity, and excellent compatibility. However, challenges such as electrode passivation, nonspecific adsorption, signal drift, and result accuracy need further refinement. Moreover, more sensitive and specific biomarkers are necessary for the initial diagnosis of CRC, along with the combinations of biomarkers for multiple tests. This study also presents an updated survey of electrochemical-cell-based biosensors, demonstrating promise for cancer detection.
Keywords: Colorectal cancer, Electrochemical biosensors, Nanomaterials, Immunosensors
Copyright and License Information
© 2024 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
This study was self-funded by the authors and received no external financial support from any funding organization.
Introduction
Cancer happens as the fault or atypical control of the cellular pathways, including cell growth, differentiation, and death.1 Colorectal cancer (CRC) is one of the most common cancers. This kind of cancer is a significant threat to our lives and is the fourth cause of cancer-related death.2,3 The fundamental causes of the growing prevalence of CRC are elderliness, nutritional conditions, and lifestyle-associated risk factors such as obesity, low physical activity, and smoking.4 For effective treatment, it is essential to monitor cancer cells swiftly, precisely, and sensitively. Moreover, further and better scrutinizing methods are required for cancer treatment.5
The progressive stage of CRC is used to diagnose the majority of patients. It indicates that tumor cells have spread and produced secondary cancers, which have a high mortality rate. Compared to CRC patients in the primary stage, who have a 90% chance of surviving for 5 years, those in the progressive stage have a 5-year survival rate of only 5-10%.6 Therefore, the primary finding of CRC will decrease its related mortality, providing more intervention capacity and treatment chance. Presently, techniques that are utilized to primarily find and predict cancers include Western blotting, enzyme-linked immunosorbent assays, and flow cytometry. Inappropriately, these methods face technical obstacles and disadvantages such as time-consuming procedures, complicated operation processes, and an essential need for sample volume. In addition, trace biomarkers might not be distinguished at the primary stage of CRC. Consequently, it is essential to develop a method without these disadvantages and barriers.7
According to Perumal and Hashim, scientists can now identify cancer cells using electrochemical biosensors.5 CRC biomarkers can be quantitatively distinguished using electrochemical biosensors. CEA, CA-50, CA-199, CA-724, p53, Kras, Braf, EGFR, mucins, interleukin, APC genes, and microRNA (miRNA) are some CRC biomarkers.2 Identifying CRC biomarkers is significant for primary diagnosis, therapeutic effect observation, disease scrutiny, prognosis, and targeted therapy.8 Additionally, electrochemical biosensors have drawn significant interest in several fields, including cancer detection and treatment.9 When creating electrochemical biosensors, altering various nanomaterials offers a quick, simple, distinctive, and sensitive method.10,11 This review study discusses several electrochemical biosensors for detecting CRC biomarkers, such as carbon materials, carcinoembryonic antigen (CEA), carbohydrate antigen, and miRNA, which are used for CRC diagnosis and prognosis.
Electrochemical Biosensors for Colorectal Cancer Marker Detection
CRC-related Biomarkers are essential for diagnosing cancers in clinical applications. Further, they are significant markers for the exact diagnosis, prognosis, and follow-up of the disease.12,13 Due to the high specificity of the biological response and the high sensitivity of the electrochemical analysis, electrochemical biosensors provide an efficient way to detect CRC markers.14 The electrochemical biosensor is the diagnostic tool that converts biochemical events such as enzyme-substrate reactions and antigen-antibody interactions to electrical signals.15,16 Electrodes are usually utilized as transformation parts in electrochemical biosensors (Figure 1). The biological identification components are typically coated on the electrode’s surface. Their response signal is converted into electrical signals inside electrodes to purposefully differentiate between biomolecules and the target substance, resulting in the qualitative or quantitative recognition of the target element.17,18
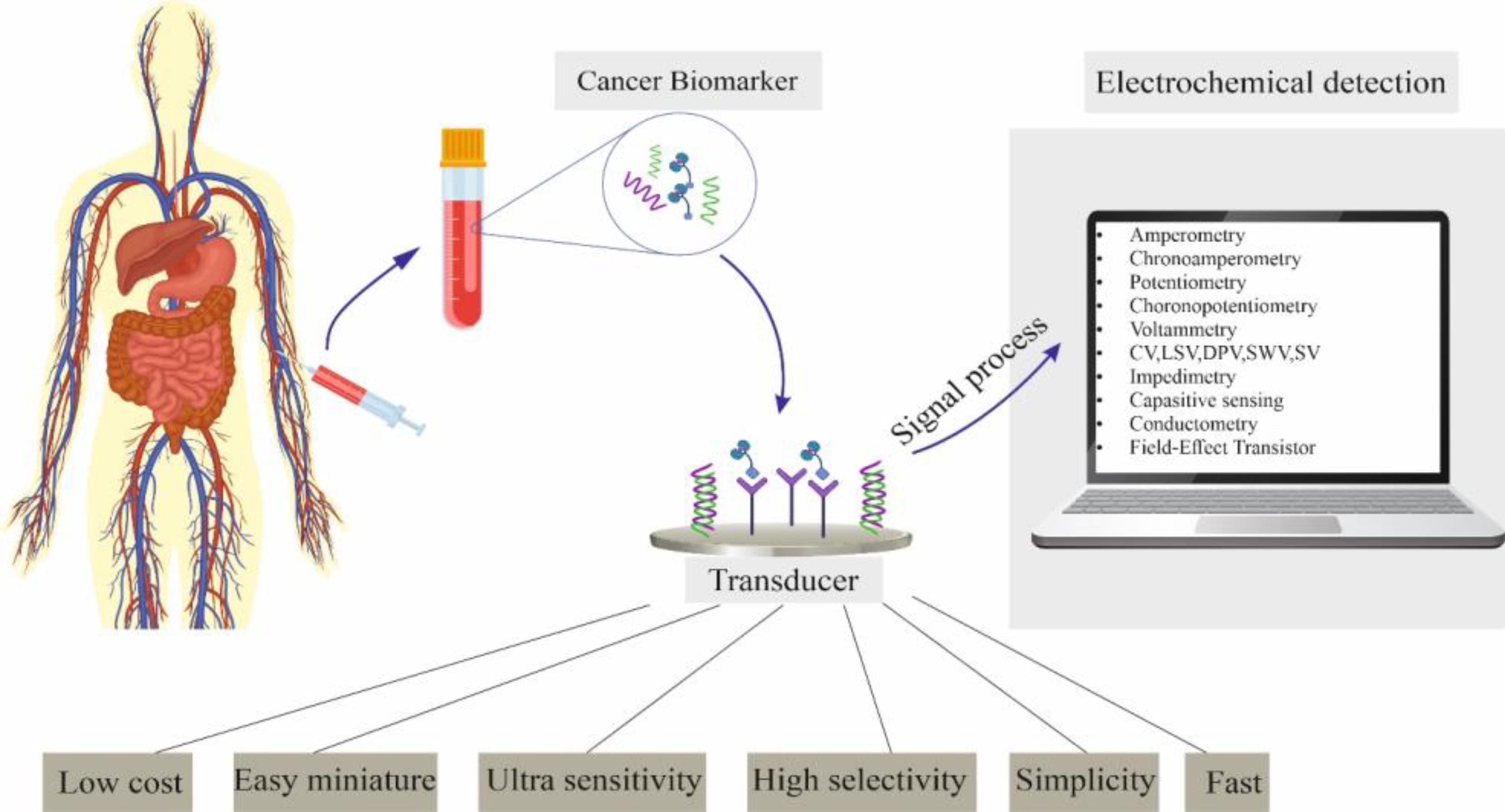
Figure 1.
Advantages, Essential Components, and Different Measurement Methods of the Electrochemical Biosensor
.
Advantages, Essential Components, and Different Measurement Methods of the Electrochemical Biosensor
A usual electrochemical biosensor contains three parts; the first part includes biomolecular identification components, which are biologically active ingredients with molecular detection abilities. They mostly encompass antibodies, nucleic acids, enzymes, aptamers (Apt), tissues, and cells that play a significant role in the practical construction of sensors.19 The transducer, or electrode, is the second component. It modifies the communication between the biometric component and the object to behave more like a slow electrical signal. The exchange circuit makes up the third component. Regarding various biological detection systems, electrochemical biosensors are categorized into enzyme sensors, immunosensors, genosensors, and aptasensors.2
The antibody is attached to the electrode’s surface in an electrochemical immunosensor. Specific binding between the antibody and the antigen results in the electrochemical reaction to different antigen concentrations in the solution, which is then performed with the redox action. Apt is a brief oligonucleotide that can bind to appropriate ligands with strong specificity and high affinity. Apt can be produced in large quantities, is easy to modify, and has developed stability compared to antibodies.20 The target single-stranded DNA molecule with a corresponding sequence is hybridized with the electrode-fixed ssDNA probe in electrochemical genosensors to detect the presence of the target ssDNA molecule in the solution. The most widely used sensors are electrochemical biosensors, which are the first advanced biosensors and are widely utilized in research content.21,22 This article examines how various CRC biomarkers have been applied to various electrochemical biosensors.
Carbon Material-Based Electrochemical Biosensors
Due to their electrical and structural characteristics, carbon-based materials can be used to make electrochemical sensors. Due to their speed and sensitivity in detecting molecules in various media, they can be utilized as nanodevices. Moreover, they show a large surface area, outstanding mechanical plasticity, electrical conductivity, and chemical and thermal stability.23 As a result, carbon-based nanomaterials are extraordinarily advantageous and have been employed in various industrial fields.24 Carbon nanomaterials, such as graphene and carbon nanotubes (CNTs), are applied to develop electrochemical biosensors.
CNTs come in two varieties, namely, single- and multi-walled CNTs (SWCNTs and MWCNTs). SWCNTs have recently been used to improve the electrical properties of biosensors. According to Singh et al, SWCNTs have many electronic and mechanical features.25 Due to their physicochemical properties, SWCNTs have drawn much attention in electrochemical biosensors.26 Large surface areas of SWCNTs have been shown to increase the immobilized enzymes’ capacity, broaden the response zones between the substrate and enzyme, simplify electrical conduction, and elevate the biosensors’ signal reaction.27 Therefore, all these features suggest that SWCNTs may be able to stimulate electron-transfer reactions in various biological molecules. In biological applications, SWCNT insolubility can present a problem. Several nanocomposites with exclusive biocompatibility features were accepted with SWCNTs to overcome constant unsolvability in aqueous mixtures.28,29
MWCNTs are fabricated of different layers of single-walled graphene cylinders whose construction is maintained by Van der Waals forces and has an interlayer space of 3.4 Å.30 According to Agü et al, the sidewall structure of MWCNTs is similar to the basal plane of graphite.31 In this respect, electron transfer rates might be similar to those of the graphite edge-plane electrode. Although MWCNTs are less remarkable than SWCNTs and MWCNTs in terms of their properties, MWCNTs are regarded as a 1-dimensional carbon system. MWCNTs, on the other hand, have been acting as a better electrode scaffold due to their superior transference and electro-catalytic properties. The antibodies’ solid substrate can be changed through chemical processing for immobilization.30
CNT-modified electrodes serve as a versatile scaffold and are used in many enzyme biosensors. However, biosensors with CNT-changed electrodes have received less attention. This is possible because immobilization methods for this modified electrode frequently involve covalent or direct sorption bonds, which can decrease the consistency of molecules and the bioelectrodes’ reproducibility.32 Alkyne-azide conjugates were created through Cu(I)-catalyzed azide-alkyne cycloaddition using MWCNTs as electrode convertors in the expansion of a transforming growth factor immunosensor.33
One of their main drawbacks is that the industrial process for CNTs is not entirely controlled. According to Kavosi et al, accumulation and poor consistency are serious issues.34 Furthermore, CNTs are typically insoluble, which limits the applications for which they are helpful. Robust van der Waals and stacking forces lead to an invariable collection phenomenon caused by MWCNTs in a watery solution, restricting their application.35 MWCNT surfaces are chemically modified with sulfonic acid, hydroxyl, and carboxyl groups to improve the dispersity and consistency of the film on electrode surfaces.36,37
Carcinoembryonic Antigen Detection
CEA is a tumor marker usually used worldwide.38 It was primarily designated in 1965 by Gold and Freedman.39 They recognized an antigen in the fetal colon and colon with adenocarcinoma; conversely, it seemed not to be present in the healthy adult colon. This protein was known as a CEA because it was identified only in cancer and embryonic tissues.40
The CEA level is typically not produced significantly after birth.41 Identifying CEA, one of the essential tumor markers, is crucial for various disease diagnoses, monitoring, and therapeutic estimation.42 Less than 5 gL-1 of CEA is typically expressed in healthy adults. More than 20 gL-1 of CEA in the blood serum may indicate cancer. Due to its high concentration in roughly 95% of CRC cases, accurate detection in the serum is crucial for the analysis and monitoring of CRC.43 Researchers developed an immunosensor based on antibody-antigen-specific binding for the detection of CEA. By using electrospinning technology, they enhanced AuNPs and MWCNTs onto the outermost core-shell nanofiber electrodes. Therefore, a specific CEA antibody was utilized on the electrode’s surface to identify CEA unmistakably. Because of the high surface-to-volume ratio and controllable porosity of the nanofibers, the strong conduction of AuNPs and MWCNTs caused a growth in the functional superficial area of electrochemical electrodes and improved electron transference.44
Jia et al designated the acquisition of a sandwich electrochemical immunosensor for CEA detection. While Au@PtPd porous nanorods (Au@PtPd MPS) served as the signal enhancer, MoS2/CuS-Au functioned as the detecting platform. The layered MoS2 and CuS transmission metal arrangement surpasses the combination phenomenon triggered by solid Van der Waals power in the MoS2 layered crystal. To comprehend the initial amplification of the signal, the Au-S link simultaneously disabled AuNPs and Ab1. The trimetallic section of the Au@PtPd MPS and its unique porous core-shell rod-like structure allowed for efficient catalytic operation. Due to its advantageous biocompatibility, the Ab2 was modified, and the sandwich reaction was accomplished. The amperometric response of CEA at various concentrations was distinguished under ideal investigational circumstances. The intended immunosensor’s range was between 50 ng mL-1 and 100 ng mL-1. Five groups of the intended electrodes were similarly used to distinguish CEA, and the relative standard deviation was less than 5%, proving the immunosensor’s advantageous reproducibility. Alpha-fetoprotein, prostate-specific antigen, and human immunoglobulin G were utilized as interference to examine the immunosensor’s selectivity. The high selectivity of the immunosensor was demonstrated by the lack of any reaction when CEA was not present in the analyte with interference. The sandwich immunosensor primarily relies on the inferred detection of secondary components, which can be sensitively developed and have a lower recognition threshold. Development in transportability and convenience is necessary because the sandwiched sensor has a complicated setup procedure and requires a lengthy reply time.45
Researchers proposed an electrochemical immunosensor for quantifiable CEA recognition using Au-Ag/rGO@PDA nanocomposites. Due to polydopamine’s exceptional reduction and self-aggregation, silver nanoparticles (AgNPs), polydopamine (PDA)-based nanomaterial, and AgNO3 were alleviated to AgNPs. The adsorption capability of fixed antibodies was improved in the presence of AuNPs. A dual signal development mechanism with Ag/rGO@PDA was also created, allowing the differentiation of the electrochemical reaction of CEA at dissimilar expressions using cyclic voltammetry, which has a linear range of 0.001 ng mL-1 to 80 ng mL-1 to find the threshold of 0.286 pg mL-1. Thereinafter, CEA was examined, but it was only 16% less than the initial result, representing good constancy.46
Feng et al used GO-AuNPs as a CEA detection platform, an electrochemical sandwich immunosensor, and Fe3O4@SiO2-NH2 as a transporter. The covalent bond that holds Ab2 and carboxyl ferrocene (Fc-COOH) together on the external side of Fe3O4@SiO2-NH2 can speed up the decomposition of H2O2. GO-AuNPs more effectively immobilize Ab1. A common approach for the sensitive discovery of CRC markers is now possible thanks to this technique; thus, developing a high-performance immunosensor has a wide range of potential applications in diagnosing CRC.47
Antigen Detection for Carbohydrates
Carbohydrate antigen (CA 19.9), also named Sialyl Lewis a, produced by gastrointestinal epithelium,48 is a tumor-associated antigen with a high molecular weight that can be released into the blood and expressed on various cancers’ cell membranes. A high molecular weight describes it. This marker is used to diagnose gastric, colorectal, and pancreatic cancers. Similar to CEA, it is not a cancer-specific marker and is related to a histological kind of carcinoma and the tissue it comes from.49
Combining CEA and CA 19.9 assays can improve diagnostic sensitivity for detecting CRC. Additionally, Stiksma et al indicated that the simultaneous detection of both markers is utilized as a postoperative factor in determining the disease’s stage and how best to treat patients.50,51 CA199 was primarily found in patients with CRC and PC in 1981.52 The typical concentration is less than 37 U mL-1 in sera. The atypical concentration could be considered one of the related situation indicators for the auxiliary prognostic and diagnosis estimation of CRC.53 Thus, several extremely sensitive electrochemical immunosensors have been constantly progressed to distinguish CA199. In addition, the CA199 and CEA detection can successfully forecast CRC relapse and metastasis.54
Metal nanoparticles are frequently applied in electrochemical biosensors because of their vast, precise surface region and high surface free energy, increasing the electrochemical catalytic capacity of the biosensor.2 Gold and silver bimetal alloy hollow nanocrystals (AuAgHNCs) were created using a technique developed by researchers. Considering the development of the catalytic flow of the oxygen-lessening response by AuAgHNCs, a novel electrochemical immunosensor was tested for the CA199 level. Because of the high biocompatibility and catalytic action of AuAgHNCs, the immunosensor presented an outstanding analytical operation for CA199 detection, with a proper linear range between 1 U mL-1 and 30 U mL-1 and a finding limit of 0.228 U mL-1. Due to their exclusive electrical conductivity and redox features, conductive polymers developed an exploration hotspot in the electrochemical field.55
A new electrochemical redox-active nanocomposite was manufactured and improved on a glassy carbon electrode for CA199 recognition.56 The co-oxidants HAuCl4 and K2PtCl4 were used in this device as co-oxidants, and the monomer N, N′-diphenyl phenylenediamine (PPPD) was applied. Due to the operational groups in their monomers, polyaniline derivatives only produce one electrochemical redox signal. After modification, the highest flow of the altered composite meaningfully rises, displaying strong electro-catalytic and electron transference abilities. In conclusion, square wave voltammetry was utilized to examine the immunosensor’s response to various CA199 expressions, and the recognition limit was 2.3 × 104 U mL-1. The creation of the sensor also provides a reference for using other nanocomposites. According to Heydari-Bafrooei and Ensafi, biosensors frequently employ several carbon-based nanomaterials, including CNTs, carbon nanoparticles, and graphene.21
Researchers designed an immunosensor with a sandwich construction that uses Au@Pd-core/shell bimetallic graphene nanocomposite (Au@Pd-Gra) as a signal booster and gold nanoparticle-functionalized porous graphene (Au-PGO) as a platform.57 A sizable superficial area is made available for the fixation of Ab1 and the facilitation of electron transference by presenting Au-PGO. The vigorous peroxidase catalytic activity of Au@Pd-Gra and the combination of Au@Pd-Gra with horseradish peroxidase significantly increased the biocatalytic activity of H2O2 in the formation of thionine. In ideal circumstances, the linear range of CA199 evaluated by differential pulse voltammetry was 0.015-150 U mL-1, and the recognition limit was 0.006 U mL-1.
MicroRNA Detection
RNA molecules with only one strand make up miRNA. It is encoded by endogenous genes, which are about 22 nucleotides long. Other genes that play a role in cell differentiation and growth can be regulated by miRNA. According to Maqbool and Hussain, miRNA expression outside of the normal range can lead to tumors and cardiovascular diseases.58
Some studies have discovered that miRNA is overexpressed, downregulated, or removed through CRC progress. Therefore, several miRNAs can be utilized as biomarkers for the diagnosis and prognosis of CRC.59,60 For instance, carcinogenic miRNAs such as miR-21 can regulate the growth of CRC by performing on target genes, developing one of the widely scrutinized miRNAs in the prognosis, diagnosis, and even treatment of CRC.61,62
The measurable recognition and examination of correlated miRNAs through various procedures play a significant role in the primary diagnosis of CRC. The present methods usually utilized to find miRNA concentration levels include real-time polymerase chain reaction (RT-PCR),63 DNA microarray,64 and northern blot technology.65 There are some difficulties even though these techniques can sensitively distinguish miRNAs. For instance, the RT-PCR technique is sensitive and requires specific primers. Due to the high cost and lack of a specific standard for information investigation and confirmation, the specificity of the microarray technique is lower than that of the RT-PCR technique. Cross-hybridization occurs when homogeneous samples are analyzed using high-sequence matches.66 The northern blot method’s low sensitivity and high sample processing requirements have led to the distinction between high sensitivity and practical techniques for its advancement. Electrochemical gene biosensors have recently advanced due to their advantages of high sensitivity, ease of use, and accessibility, as well as their favorable perspectives in target recognition and disease research.67
Due to the target miRNA’s low expression during the recognition process, a correct signal might not be attained; therefore, several techniques are needed to raise the target. Oligonucleotide development methods have always advanced due to numerous nanomaterials and enzymes to distinguish better and analyze the target.68 Using two single-stranded DNA probes and target materials for sandwich hybridization tests to distinguish miRNA-21, researchers developed an electrochemical biosensor using an alkaline phosphatase (ALP) signal. In this method, the pencil graphite electrode was changed using AuNPs, and the signal was increased while setting the thiol-terminated capture probe 1 (SH-P1). Using the first fragment of the miRNA and the additional portion with the biotin-labeled probe 2 (B-P2), SH-P1 was hybridized to form a sandwich structure. In conclusion, the modified electrode contained streptavidin and ALP. An electroactive-naphthol was created to control the oxidation reaction by catalyzing the electro-inactive-naphthyl phosphate with an enzyme. The sensor exhibits high sensitivity with a detection limit of 100 pM, opening up a novel method for intensifying other enzymes.69
Tian et al planned a label-free electrochemical miRNA sensor by employing the signal increase influence of nanomaterials. Self-assembling polypyrrole and AuNPs creates a nano-superlattice construction that improves the glassy carbon electrode, increasing the efficacy of electron transfer. Single-stranded RNA (ssRNA) was utilized in a signal examination to hybridize with the target miRNA via hydrogen bonding. Eventually, toluidine blue with the redox action was attached to intensify the signal, and the sensor displayed an excellent linear association with the range of 100 aM to 1 nM, presenting a finding limit of 78 aM.70
The approaches based on oligonucleotide intensification mostly contain rolling circle amplification (RCA), hybridization chain reaction (HCR), and catalytic hairpin assembly (CHA). RCA is a method in which circular DNA is employed as a pattern, generated by a DNA primer, and converted into single-stranded DNA through enzyme catalysis. The circular pattern has more benefits in sensitivity and specificity because of its firm link and effective polymerization.71
HCR is a signal increase method that exhibits an isothermal enzyme-free feature in which the hybridization of two connected and matching molecular probes is started by the starting chain to shape long strands of DNA with nicks in the double helix.72 CHA is an uncomplicated isothermal amplification method. In the existence of stimulators, two hairpins hybridize to produce a double strand that causes catalytic intensification.73
To differentiate miRNA-21, researchers devised an electrochemical biosensor that would take advantage of CHA and RCA in the form of a double signal intensification strategy 74. The thiolated probe was initially presented on the gold electrode’s surface, and biotin-labeled H1 and H2 hybrid double strands were generated to detect miRNA-21. Subsequently, the RCA was detected through DNA polymerase activity, and its output was synchronized with streptavidin-labeled ALP to generate electrical signals. The final results of differential pulse voltammetry analysis demonstrated a positive linear correlation with a range of 0.5 pM to 12500 pM and a detection limit of 290 fM. Liang et al demonstrated that the cascade hybridization chain reaction (C cascade HCR) enhances an electrochemical biosensor for detecting miRNA-21.75 Using this strategy, the downstream HCR’s input is derived from the upstream HCR’s intermediate creation. With a recognition limit of 11 pM, miRNA-21 can distinguish highly homologous targets and initiate DNA self-assembly via the chain movement response. In the study performed by Cheng et al, an electrochemical biosensor was designed to find miRNA-21 using the CHA/HCR intensification strategy.76
Conclusion
The prompt emphasizes the significance of early tumor cell diagnosis for cancer patients’ effective treatment and recovery. It underscores the need for simple and sensitive diagnostic procedures to identify multiple tumor biomarkers at low bodily fluid concentrations. The study discussed the potential of electrochemical-cell-based biosensors as a promising alternative to traditional cancer detection methods, highlighting their high sensitivity, adaptability, and rapid response in identifying biomarkers associated with CRC. It also highlighted the advantages of nanomaterials, surface functionalization, and high electrochemical activity in constructing electrochemical biosensors. The study concludes by urging further development to address challenges such as electrode passivation, nonspecific adsorption, drift in sensor capabilities, and the need for more sensitive and specific biomarkers for the initial diagnosis of CRC, as well as the integration of nanomaterials, sensor arrays, and microfluidic chips in future research efforts to create efficient and rapid biological recognition systems.
Authors’ Contribution
Conceptualization: Zheng Dong, Zheifeng Xu.
Data curation: Zheng Dong, Zheifeng Xu.
Formal analysis: Zheng Dong, Zheifeng Xu.
Funding acquisition: Zheng Dong, Zheifeng Xu.
Investigation: Zheng Dong, Zheifeng Xu.
Methodology: Zheng Dong, Zheifeng Xu.
Project administration: Zheng Dong, Zheifeng Xu.
Resources: Zheng Dong, Zheifeng Xu.
Software: Zheng Dong, Zheifeng Xu.
Supervision: Zheifeng Xu.
Validation: Zheng Dong, Zheifeng Xu.
Visualization: Zheng Dong, Zheifeng Xu.
Writing–original draft: Zheng Dong, Zheifeng Xu.
Writing–review & editing: Zheng Dong, Zheifeng Xu.
Competing Interests
The authors declare that they have no conflict of interests.
Ethical Approval
Not applicable.
References
- Mohammed RN, Khosravi M, Rahman HS, Adili A, Kamali N, Soloshenkov PP. Anastasis: cell recovery mechanisms and potential role in cancer. Cell Commun Signal 2022; 20(1):81. doi: 10.1186/s12964-022-00880-w [Crossref] [ Google Scholar]
- Zhang W, Xiao G, Chen J, Wang L, Hu Q, Wu J. Electrochemical biosensors for measurement of colorectal cancer biomarkers. Anal Bioanal Chem 2021; 413(9):2407-28. doi: 10.1007/s00216-021-03197-8 [Crossref] [ Google Scholar]
- Akbari M, Adili A, Faraji A, Pakdel A, Aslaminabad R, Nasrabadi D. Restoration of miR-124 serves as a promising therapeutic approach in CRC by affecting CDK6 which is itself a prognostic and diagnostic factor. Gene Rep 2021; 24:101274. doi: 10.1016/j.genrep.2021.101274 [Crossref] [ Google Scholar]
- Akbari M, Shanehbandi D, Asadi M, Shomali N, Faraji A, Khaze V. Effects of CD133 silencing on survival and migration of HT-29 colorectal cancer cells. Iran J Immunol 2019; 16(3):246-57. doi: 10.22034/iji.2019.80275 [Crossref] [ Google Scholar]
- Perumal V, Hashim U. Advances in biosensors: principle, architecture and applications. J Appl Biomed 2014; 12(1):1-15. doi: 10.1016/j.jab.2013.02.001 [Crossref] [ Google Scholar]
- Favoriti P, Carbone G, Greco M, Pirozzi F, Pirozzi RE, Corcione F. Worldwide burden of colorectal cancer: a review. Updates Surg 2016; 68(1):7-11. doi: 10.1007/s13304-016-0359-y [Crossref] [ Google Scholar]
- Metkar SK, Girigoswami K. Diagnostic biosensors in medicine–a review. Biocatal Agric Biotechnol 2019; 17:271-83. doi: 10.1016/j.bcab.2018.11.029 [Crossref] [ Google Scholar]
- Tarney CM, Wang G, Bateman NW, Conrads KA, Zhou M, Hood BL, et al. Biomarker panel for early detection of endometrial cancer in the prostate, lung, colorectal, and ovarian cancer screening trial. Am J Obstet Gynecol 2019;221(5):472.e1-472.e10. 10.1016/j.ajog.2019.06.005.
- Zhou H, Du X, Zhang Z. Electrochemical sensors for detection of markers on tumor cells. Int J Mol Sci 2021; 22(15):8184. doi: 10.3390/ijms22158184 [Crossref] [ Google Scholar]
- Wang J. Nanomaterial-based electrochemical biosensors. Analyst 2005; 130(4):421-6. doi: 10.1039/b414248a [Crossref] [ Google Scholar]
- Filik H, Avan AA. Nanostructures for nonlabeled and labeled electrochemical immunosensors: simultaneous electrochemical detection of cancer markers: a review. Talanta 2019; 205:120153. doi: 10.1016/j.talanta.2019.120153 [Crossref] [ Google Scholar]
- Ganepola GA, Nizin J, Rutledge JR, Chang DH. Use of blood-based biomarkers for early diagnosis and surveillance of colorectal cancer. World J Gastrointest Oncol 2014; 6(4):83-97. doi: 10.4251/wjgo.v6.i4.83 [Crossref] [ Google Scholar]
- Patel JN, Fong MK, Jagosky M. Colorectal cancer biomarkers in the era of personalized medicine. J Pers Med 2019; 9(1):3. doi: 10.3390/jpm9010003 [Crossref] [ Google Scholar]
- Rezaei B, Irannejad N. Electrochemical detection techniques in biosensor applications. In: Ensafi AA, ed. Electrochemical Biosensors. Elsevier; 2019. p. 11-43. 10.1016/b978-0-12-816491-4.00002-4.
- Li H, Liu X, Li L, Mu X, Genov R, Mason AJ. CMOS electrochemical instrumentation for biosensor microsystems: a review. Sensors (Basel) 2016; 17(1):74. doi: 10.3390/s17010074 [Crossref] [ Google Scholar]
- Zheng H, Ma X, Chen L, Lin Z, Guo L, Qiu B. Label-free electrochemical impedance biosensor for sequence-specific recognition of double-stranded DNA. Anal Methods 2013; 5(19):5005-9. doi: 10.1039/c3ay40972d [Crossref] [ Google Scholar]
- Ronkainen NJ, Halsall HB, Heineman WR. Electrochemical biosensors. Chem Soc Rev 2010; 39(5):1747-63. doi: 10.1039/b714449k [Crossref] [ Google Scholar]
- Hammond Jules L, Formisano N, Estrela P, Carrara S, Tkac J. Electrochemical biosensors and nanobiosensors. Essays Biochem 2016; 60(1):69-80. doi: 10.1042/ebc20150008 [Crossref] [ Google Scholar]
- Justino CI, Freitas AC, Pereira R, Duarte AC, Rocha Santos TA. Recent developments in recognition elements for chemical sensors and biosensors. Trends Analyt Chem 2015; 68:2-17. doi: 10.1016/j.trac.2015.03.006 [Crossref] [ Google Scholar]
- Khanmohammadi A, Aghaie A, Vahedi E, Qazvini A, Ghanei M, Afkhami A. Electrochemical biosensors for the detection of lung cancer biomarkers: a review. Talanta 2020; 206:120251. doi: 10.1016/j.talanta.2019.120251 [Crossref] [ Google Scholar]
- Heydari-Bafrooei E, Ensafi AA. Typically used carbon-based nanomaterials in the fabrication of biosensors. In: Ensafi AA, ed. Electrochemical Biosensors. Elsevier; 2019. p. 77-98. 10.1016/b978-0-12-816491-4.00004-8.
- Farbod F, Mazloum-Ardakani M. Typically used nanomaterials-based noncarbon materials in the fabrication of biosensors. In: Ensafi AA, ed. Electrochemical Biosensors. Elsevier; 2019. p. 99-133. 10.1016/b978-0-12-816491-4.00005-x.
- Beluomini MA, da Silva JL, de Sá AC, Buffon E, Pereira TC, Stradiotto NR. Electrochemical sensors based on molecularly imprinted polymer on nanostructured carbon materials: a review. J Electroanal Chem (Lausanne) 2019; 840:343-66. doi: 10.1016/j.jelechem.2019.04.005 [Crossref] [ Google Scholar]
- Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017; 66(4):683-91. doi: 10.1136/gutjnl-2015-310912 [Crossref] [ Google Scholar]
- Singh P, Campidelli S, Giordani S, Bonifazi D, Bianco A, Prato M. Organic functionalisation and characterisation of single-walled carbon nanotubes. Chem Soc Rev 2009; 38(8):2214-30. doi: 10.1039/b518111a [Crossref] [ Google Scholar]
- Guo X. Single-molecule electrical biosensors based on single-walled carbon nanotubes. Adv Mater 2013; 25(25):3397-408. doi: 10.1002/adma.201301219 [Crossref] [ Google Scholar]
- Zhang X, Li CR, Wang WC, Xue J, Huang YL, Yang XX. A novel electrochemical immunosensor for highly sensitive detection of aflatoxin B1 in corn using single-walled carbon nanotubes/chitosan. Food Chem 2016; 192:197-202. doi: 10.1016/j.foodchem.2015.06.044 [Crossref] [ Google Scholar]
- Saleh Ahammad AJ, Lee JJ, Rahman MA. Electrochemical sensors based on carbon nanotubes. Sensors (Basel) 2009; 9(4):2289-319. doi: 10.3390/s90402289 [Crossref] [ Google Scholar]
- Pan J, Li F, Choi JH. Single-walled carbon nanotubes as optical probes for bio-sensing and imaging. J Mater Chem B 2017; 5(32):6511-22. doi: 10.1039/c7tb00748e [Crossref] [ Google Scholar]
- Zhou Y, Fang Y, Ramasamy RP. Non-covalent functionalization of carbon nanotubes for electrochemical biosensor development. Sensors (Basel) 2019; 19(2):392. doi: 10.3390/s19020392 [Crossref] [ Google Scholar]
- Agüí L, Yáñez-Sedeño P, Pingarrón JM. Role of carbon nanotubes in electroanalytical chemistry: a review. Anal Chim Acta 2008; 622(1-2):11-47. doi: 10.1016/j.aca.2008.05.070 [Crossref] [ Google Scholar]
- Cho IH, Kim DH, Park S. Electrochemical biosensors: perspective on functional nanomaterials for on-site analysis. Biomater Res 2020; 24:6. doi: 10.1186/s40824-019-0181-y [Crossref] [ Google Scholar]
- Sánchez-Tirado E, González-Cortés A, Yáñez-Sedeño P, Pingarrón JM. Carbon nanotubes functionalized by click chemistry as scaffolds for the preparation of electrochemical immunosensors Application to the determination of TGF-beta 1 cytokine. Analyst 2016; 141(20):5730-7. doi: 10.1039/c6an00941g [Crossref] [ Google Scholar]
- Kavosi B, Salimi A, Hallaj R, Amani K. A highly sensitive prostate-specific antigen immunosensor based on gold nanoparticles/PAMAM dendrimer loaded on MWCNTS/chitosan/ionic liquid nanocomposite. Biosens Bioelectron 2014; 52:20-8. doi: 10.1016/j.bios.2013.08.012 [Crossref] [ Google Scholar]
- Li M, Wang P, Li F, Chu Q, Li Y, Dong Y. An ultrasensitive sandwich-type electrochemical immunosensor based on the signal amplification strategy of mesoporous core–shell Pd@Pt nanoparticles/amino group functionalized graphene nanocomposite. Biosens Bioelectron 2017; 87:752-9. doi: 10.1016/j.bios.2016.08.076 [Crossref] [ Google Scholar]
- Wei Y, Ling X, Zou L, Lai D, Lu H, Xu Y. A facile approach toward preparation of sulfonated multi-walled carbon nanotubes and their dispersibility in various solvents. Colloids Surf A Physicochem Eng Asp 2015; 482:507-13. doi: 10.1016/j.colsurfa.2015.07.005 [Crossref] [ Google Scholar]
- Singh C, Srivastava S, Ali MA, Gupta TK, Sumana G, Srivastava A. Carboxylated multiwalled carbon nanotubes-based biosensor for aflatoxin detection. Sens Actuators B Chem 2013; 185:258-64. doi: 10.1016/j.snb.2013.04.040 [Crossref] [ Google Scholar]
- Duffy MJ. Carcinoembryonic antigen as a marker for colorectal cancer: is it clinically useful?. Clin Chem 2001; 47(4):624-30. [ Google Scholar]
- Gold P, Freedman SO. Specific carcinoembryonic antigens of the human digestive system. J Exp Med 1965; 122(3):467-81. doi: 10.1084/jem.122.3.467 [Crossref] [ Google Scholar]
- Boucher D, Cournoyer D, Stanners CP, Fuks A. Studies on the control of gene expression of the carcinoembryonic antigen family in human tissue. Cancer Res 1989; 49(4):847-52. [ Google Scholar]
- Hall C, Clarke L, Pal A, Buchwald P, Eglinton T, Wakeman C. A review of the role of carcinoembryonic antigen in clinical practice. Ann Coloproctol 2019; 35(6):294-305. doi: 10.3393/ac.2019.11.13 [Crossref] [ Google Scholar]
- Xiang W, Lv Q, Shi H, Xie B, Gao L. Aptamer-based biosensor for detecting carcinoembryonic antigen. Talanta 2020; 214:120716. doi: 10.1016/j.talanta.2020.120716 [Crossref] [ Google Scholar]
- Han J, Li Y, Feng J, Li M, Wang P, Chen Z. A novel sandwich-type immunosensor for detection of carcino-embryonic antigen using silver hybrid multiwalled carbon nanotubes/manganese dioxide. J Electroanal Chem (Lausanne) 2017; 786:112-9. doi: 10.1016/j.jelechem.2017.01.021 [Crossref] [ Google Scholar]
- Paimard G, Shahlaei M, Moradipour P, Akbari H, Jafari M, Arkan E. An Impedimetric Immunosensor modified with electrospun core-shell nanofibers for determination of the carcinoma embryonic antigen. Sens Actuators B Chem 2020; 311:127928. doi: 10.1016/j.snb.2020.127928 [Crossref] [ Google Scholar]
- Jia Y, Li Y, Zhang S, Wang P, Liu Q, Dong Y. Mulberry-like Au@PtPd porous nanorods composites as signal amplifiers for sensitive detection of CEA. Biosens Bioelectron 2020; 149:111842. doi: 10.1016/j.bios.2019.111842 [Crossref] [ Google Scholar]
- Yang Y, Jiang M, Cao K, Wu M, Zhao C, Li H. An electrochemical immunosensor for CEA detection based on Au-Ag/rGO@PDA nanocomposites as integrated double signal amplification strategy. Microchem J 2019; 151:104223. doi: 10.1016/j.microc.2019.104223 [Crossref] [ Google Scholar]
- Feng T, Qiao X, Wang H, Sun Z, Hong C. A sandwich-type electrochemical immunosensor for carcinoembryonic antigen based on signal amplification strategy of optimized ferrocene functionalized Fe₃O₄@SiO₂ as labels. Biosens Bioelectron 2016; 79:48-54. doi: 10.1016/j.bios.2015.11.001 [Crossref] [ Google Scholar]
- Thomsen M, Skovlund E, Sorbye H, Bolstad N, Nustad KJ, Glimelius B. Prognostic role of carcinoembryonic antigen and carbohydrate antigen 19-9 in metastatic colorectal cancer: a BRAF-mutant subset with high CA 19-9 level and poor outcome. Br J Cancer 2018; 118(12):1609-16. doi: 10.1038/s41416-018-0115-9 [Crossref] [ Google Scholar]
- Vukobrat-Bijedic Z, Husic-Selimovic A, Sofic A, Bijedic N, Bjelogrlic I, Gogov B. Cancer antigens (CEA and CA 19-9) as markers of advanced stage of colorectal carcinoma. Med Arch 2013; 67(6):397-401. doi: 10.5455/medarh.2013.67.397-401 [Crossref] [ Google Scholar]
- Stiksma J, Grootendorst DC, van der Linden PW. CA 19-9 as a marker in addition to CEA to monitor colorectal cancer. Clin Colorectal Cancer 2014; 13(4):239-44. doi: 10.1016/j.clcc.2014.09.004 [Crossref] [ Google Scholar]
- Jelski W, Mroczko B. Biochemical markers of colorectal cancer - present and future. Cancer Manag Res 2020; 12:4789-97. doi: 10.2147/cmar.S253369 [Crossref] [ Google Scholar]
- Koprowski H, Herlyn M, Steplewski Z, Sears HF. Specific antigen in serum of patients with colon carcinoma. Science 1981; 212(4490):53-5. doi: 10.1126/science.6163212 [Crossref] [ Google Scholar]
- Nakayama T, Watanabe M, Teramoto T, Kitajima M. CA 19-9 as a predictor of recurrence in patients with colorectal cancer. J Surg Oncol 1997; 66(4):238-43. doi: 10.1002/(sici)1096-9098(199712)66:4<238::aid-jso3>3.0.co;2-c [Crossref] [ Google Scholar]
- Xu X, Niu M, Zhang B, Chang J, Su F, Wang K. Clinical value of CEA and CA 19-9 in colorectal cancer by Kaplan-Meier survival curve. Int J Clin Exp Med 2019; 12(12):13305-10. [ Google Scholar]
- Wang R, Feng JJ, Liu WD, Jiang LY, Wang AJ. A novel label-free electrochemical immunosensor based on the enhanced catalytic currents of oxygen reduction by AuAg hollow nanocrystals for detecting carbohydrate antigen 199. Biosens Bioelectron 2017; 96:152-8. doi: 10.1016/j.bios.2017.05.007 [Crossref] [ Google Scholar]
- Wang L, Shan J, Feng F, Ma Z. Novel redox species polyaniline derivative-Au/Pt as sensing platform for label-free electrochemical immunoassay of carbohydrate antigen 199. Anal Chim Acta 2016; 911:108-13. doi: 10.1016/j.aca.2016.01.016 [Crossref] [ Google Scholar]
- Yang F, Yang Z, Zhuo Y, Chai Y, Yuan R. Ultrasensitive electrochemical immunosensor for carbohydrate antigen 19-9 using Au/porous graphene nanocomposites as platform and Au@Pd core/shell bimetallic functionalized graphene nanocomposites as signal enhancers. Biosens Bioelectron 2015; 66:356-62. doi: 10.1016/j.bios.2014.10.066 [Crossref] [ Google Scholar]
- Maqbool R, Ul Hussain M. MicroRNAs and human diseases: diagnostic and therapeutic potential. Cell Tissue Res 2014; 358(1):1-15. doi: 10.1007/s00441-013-1787-3 [Crossref] [ Google Scholar]
- Shirafkan N, Mansoori B, Mohammadi A, Shomali N, Ghasbi M, Baradaran B. MicroRNAs as novel biomarkers for colorectal cancer: new outlooks. Biomed Pharmacother 2018; 97:1319-30. doi: 10.1016/j.biopha.2017.11.046 [Crossref] [ Google Scholar]
- Ng EK, Chong WW, Jin H, Lam EK, Shin VY, Yu J. Differential expression of microRNAs in plasma of patients with colorectal cancer: a potential marker for colorectal cancer screening. Gut 2009; 58(10):1375-81. doi: 10.1136/gut.2008.167817 [Crossref] [ Google Scholar]
- Marcuello M, Vymetalkova V, Neves RP, Duran-Sanchon S, Vedeld HM, Tham E. Circulating biomarkers for early detection and clinical management of colorectal cancer. Mol Aspects Med 2019; 69:107-22. doi: 10.1016/j.mam.2019.06.002 [Crossref] [ Google Scholar]
- Li T, Leong MH, Harms B, Kennedy G, Chen L. MicroRNA-21 as a potential colon and rectal cancer biomarker. World J Gastroenterol 2013; 19(34):5615-21. doi: 10.3748/wjg.v19.i34.5615 [Crossref] [ Google Scholar]
- Kroh EM, Parkin RK, Mitchell PS, Tewari M. Analysis of circulating microRNA biomarkers in plasma and serum using quantitative reverse transcription-PCR (qRT-PCR). Methods 2010; 50(4):298-301. doi: 10.1016/j.ymeth.2010.01.032 [Crossref] [ Google Scholar]
- Jiang L, Wu CL, Wu JZ, Yang X, Wang HL, Li GJ. Microarray-based measurement of microRNA-449c-5p levels in hepatocellular carcinoma and bioinformatic analysis of potential signaling pathways. Pathol Res Pract 2019; 215(1):68-81. doi: 10.1016/j.prp.2018.10.007 [Crossref] [ Google Scholar]
- He SL, Green R. Northern blotting. Methods Enzymol 2013; 530:75-87. doi: 10.1016/b978-0-12-420037-1.00003-8 [Crossref] [ Google Scholar]
- Kilic T, Erdem A, Ozsoz M, Carrara S. microRNA biosensors: Opportunities and challenges among conventional and commercially available techniques. Biosens Bioelectron 2018; 99:525-46. doi: 10.1016/j.bios.2017.08.007 [Crossref] [ Google Scholar]
- Hamidi-Asl E, Palchetti I, Hasheminejad E, Mascini M. A review on the electrochemical biosensors for determination of microRNAs. Talanta 2013; 115:74-83. doi: 10.1016/j.talanta.2013.03.061 [Crossref] [ Google Scholar]
- Mohammadi H, Yammouri G, Amine A. Current advances in electrochemical genosensors for detecting microRNA cancer markers. Curr Opin Electrochem 2019; 16:96-105. doi: 10.1016/j.coelec.2019.04.030 [Crossref] [ Google Scholar]
- Mandli J, Mohammadi H, Amine A. Electrochemical DNA sandwich biosensor based on enzyme amplified microRNA-21 detection and gold nanoparticles. Bioelectrochemistry 2017; 116:17-23. doi: 10.1016/j.bioelechem.2017.03.002 [Crossref] [ Google Scholar]
- Tian L, Qian K, Qi J, Liu Q, Yao C, Song W. Gold nanoparticles superlattices assembly for electrochemical biosensor detection of microRNA-21. Biosens Bioelectron 2018; 99:564-70. doi: 10.1016/j.bios.2017.08.035 [Crossref] [ Google Scholar]
- Xu L, Duan J, Chen J, Ding S, Cheng W. Recent advances in rolling circle amplification-based biosensing strategies-a review. Anal Chim Acta 2021; 1148:238187. doi: 10.1016/j.aca.2020.12.062 [Crossref] [ Google Scholar]
- Zhang C, Chen J, Sun R, Huang Z, Luo Z, Zhou C. The recent development of hybridization chain reaction strategies in biosensors. ACS Sens 2020; 5(10):2977-3000. doi: 10.1021/acssensors.0c01453 [Crossref] [ Google Scholar]
- Cui Y, Fan S, Yuan Z, Song M, Hu J, Qian D. Ultrasensitive electrochemical assay for microRNA-21 based on CRISPR/Cas13a-assisted catalytic hairpin assembly. Talanta 2021; 224:121878. doi: 10.1016/j.talanta.2020.121878 [Crossref] [ Google Scholar]
- Li Q, Zeng F, Lyu N, Liang J. Highly sensitive and specific electrochemical biosensor for microRNA-21 detection by coupling catalytic hairpin assembly with rolling circle amplification. Analyst 2018; 143(10):2304-9. doi: 10.1039/c8an00437d [Crossref] [ Google Scholar]
- Liang M, Pan M, Hu J, Wang F, Liu X. Electrochemical biosensor for microRNA detection based on cascade hybridization chain reaction. ChemElectroChem 2018; 5(10):1380-6. doi: 10.1002/celc.201800255 [Crossref] [ Google Scholar]
- Cheng H, Li W, Duan S, Peng J, Liu J, Ma W. Mesoporous silica containers and programmed catalytic hairpin assembly/hybridization chain reaction based electrochemical sensing platform for microRNA ultrasensitive detection with low background. Anal Chem 2019; 91(16):10672-8. doi: 10.1021/acs.analchem.9b01947 [Crossref] [ Google Scholar]