Biomed Res Bull. 2(3):140-161.
doi: 10.34172/biomedrb.2024.21
Review Article
How Oxidative Stress Modifies the Immune Response Landscape in Cancer
Somayeh Ashrafi 1, 2, 3  , Neda Zahmatkesh 4, Melika Khanzadeh Tehrani 5, Nastaran Hadizadeh 6, 7, Sara Rahimzadeh 1, 2, Mehdi Mahdavi 1, 2, 8, Mohammad Abdollahi 6, 7, *
, Neda Zahmatkesh 4, Melika Khanzadeh Tehrani 5, Nastaran Hadizadeh 6, 7, Sara Rahimzadeh 1, 2, Mehdi Mahdavi 1, 2, 8, Mohammad Abdollahi 6, 7, *  , Mohammad Hossein Yazdi 1, 2, 9, *
, Mohammad Hossein Yazdi 1, 2, 9, * 
Author information:
1
Immunotherapy Group, Pharmaceutical Sciences Research Center (PSRC), The Institute of Pharmaceutical Sciences (TIPS),
Tehran University of Medical Sciences, Tehran, Iran
2
Recombinant Vaccine Research Center, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
3
Department of Molecular and Cell Biology, Faculty of Advanced Sciences & Technology, Tehran Medical Sciences, Islamic Azad
University (IAU), Tehran, Iran
4
Department of Genetics, Zanjan Branch, Islamic Azad University, Zanjan, Iran
5
Department of Pathobiology, Faculty of Public Health, Tehran University of Medical Sciences, Tehran, Iran
6
Department of Toxicology and Pharmacology, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
7
Toxicology and Diseases Group, Pharmaceutical Sciences Research Center (PSRC), Tehran University of Medical Sciences,
Tehran, Iran
8
Advanced Therapy Medical Product (ATMP) Department, Breast Cancer Research Center, Motamed Cancer Institute, Academic
Center for Education, Culture and Research (ACECR), Tehran, Iran
9
Biotechnology Research Center, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
Abstract
There is an increasing body of evidence which proves that excessive production of toxic reactive species, primarily reactive oxygen species (ROS), damages many biomolecules, cell structures, and functions. They participate in various human pathological processes, particularly aging, neurodegenerative disorders, uncontrolled inflammation, and cancer. Inflammation is a biological response of the host’s immune system, accompanied by the involvement of various immune cells, blood vessels, and molecular mediators. Therefore, in this review, we discussed the relationship between inflammation and oxidative stress and their relationship with cancer. We also discussed the role of the immune system in inflammation, oxidative stress, and cancer. Undoubtedly, further studies about ROS and inflammatory interactions in the stimulation of immune responses can open up new horizons for researchers to design an innovative immunotherapeutic strategy for chronic inflammatory diseases and cancer-related inflammation (CRI).
Keywords: Activator protein, Antigen-presenting cell, Cyclooxygenase-2
Copyright and License Information
© 2024 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
This study was self-funded by the authors and received no external financial support from any funding organization.
Introduction
Reactive oxidants, or oxidizing agents, are defined as free radicals or unstable atoms/molecules with unpaired electrons, exhibiting a strong tendency to pair these electrons for stability. Due to their high reactivity, they readily accept an electron from electron donors or reducing agents.1,2 Under normal physiological conditions and homeostasis, non-destructive concentrations of free radicals are tightly regulated by antioxidant agents.3 However, excessive production or uncontrolled accumulation of these reactive species can directly or indirectly damage essential biomolecules, activating molecular and cellular pathways linked to inflammation and carcinogenesis.3-7 Consequently, reactive oxidants may exert beneficial or harmful effects depending on their concentration.8,9 Reactive species can originate from endogenous sources, including growth factors, cytokines, metabolic processes, and immune cells,10,11 or from exogenous factors such as dietary nutrients, UV rays, the microbiome, and environmental xenobiotics. Both endogenous and exogenous sources contribute significantly to oxidative damage of biomolecules (e.g., DNA, lipids, and proteins), ultimately leading to reactive oxygen species (ROS) accumulation, oxidative stress, and potential neoplastic transformation.3,10,12-14
In biological systems and clinical settings, four primary classes of reactive species are recognized based on their structural composition15:
simple
-
-
Reactive nitrogen species (RNS)
-
Reactive sulfur species (RSS)
-
Reactive chlorine species (RCS)
Reactive species encompass both radical and non-radical forms.16 For instance, RNS include radicals like nitric oxide (NO∙) and non-radicals such as peroxynitrite (ONOO−), whereas RCS consist of atomic chlorine (Cl∙) and hypochlorous acid (HOCl) as the non-radical form.17,18 Among reactive species, nitrogen and oxygen radicals, especially superoxide anions (O2∙−) and ONOO−, are highly reactive and carcinogenic, produced via both endogenous and exogenous pathways that induce oxidative stress, inflammation, and tumor development.1,17,19-21
Inflammation, the body’s natural response to endogenous and exogenous stimuli, is characterized by the recruitment and infiltration of inflammatory cells into target tissues, aiming to eliminate harmful stimuli, repair damage, and restore tissue homeostasis. This inflammatory response persists until pathogens are eradicated, homeostasis is reestablished, and tissue repair mechanisms are activated.22,23
Inflammatory responses involve innate and adaptive immunity components, including phagocytes (neutrophils/macrophages), dendritic cells (DCs), mast cells, eosinophils, leukocytes, monocytes, natural killer cells, lymphocytes, and various pro-inflammatory molecules such as cytokines, chemokines, proteases, and reactive nitrogen-oxygen species (RONS).24-26
During inflammation, antioxidant and cytoprotective mechanisms support redox homeostasis by activating genes involved in tissue repair.27 Unchecked inflammatory responses, however, can cause tissue damage and cellular hyperplasia due to excessive RONS production by inflammatory cells, which disrupts antioxidant pathways,28 leading to oxidative stress.29
Cellular and extracellular redox buffering systems, which consist of key enzymatic antioxidants (e.g., catalase [CAT], superoxide dismutase [SOD], nitric oxide synthases [NOSs], glutathione peroxidase [GPx], and thioredoxin reductase [TR]) and non-enzymatic antioxidants (e.g., ascorbate (vitamin C), β-carotene, α-tocopherol (vitamin E), and flavonoids), play a crucial role in maintaining ROS/RNS at safe levels.3,30-32 Both enzymatic and non-enzymatic antioxidants work to protect biomolecules by reducing RONS levels. Antioxidants further mitigate inflammation by lowering the levels of nuclear factor kappa B (NF-κB), mitogen-activated protein kinase (MAPK), cytokines, and nitric oxide, commonly utilized to defend against inflammation and cancer. Conversely, high doses of exogenous antioxidants can be toxic, exerting pro-oxidative, pro-nitrosative, pro-glycation, and pro-inflammatory effects.33-35 Imbalances in RONS and antioxidant production that disrupt redox signaling can trigger pro-inflammatory signaling pathways, resulting in the oxidation of biomolecules such as proteins, phospholipids, carbohydrates, and nucleic acids, ultimately contributing to cellular degeneration, genomic instability, mutation, and functional decline.36,37
Inflammatory Conditions: Basic Concepts
Acute Inflammatory Condition
Inflammation is broadly classified into two types: acute and chronic. Each type initiates specific signaling pathways to stimulate both innate and adaptive immune responses.36,38 Acute inflammation represents the body’s initial response to harmful stimuli, characterized by the activation of innate non-specific immunity. This process is triggered when pathogen-associated molecular patterns (PAMPs) are detected by pattern recognition receptors (PRRs), particularly those in the Toll-like receptor (TLR) family.39 TLR-4, which is specifically activated by lipopolysaccharides (LPS) and other pathogen-related molecules, plays a critical role in activating innate immune responses and inducing acute inflammation and oxidative stress.22,40 Granulocytes are the predominant inflammatory cells in acute inflammation, mobilized to the site of infection or injury.41,42 Various innate immune cells, such as DCs, macrophages, neutrophils, fibroblasts, mast cells, and circulating leukocytes, express PRRs to recognize PAMPs and respond to bacterial components like LPS, lipoteichoic acid, or peptidoglycans, leading to acute inflammatory responses.43-45
The initial acute inflammatory response includes the degranulation of neutrophils and platelets, followed by the activation of tissue macrophages 45. In addition, acute inflammation activates DCs, which function as professional antigen-presenting cells (APCs), enabling them to capture and process antigens for presentation to naive T cells. Activated macrophages and DCs subsequently release various inflammatory mediators, including immunoregulatory cytokines, chemokines, anti-inflammatory lipid mediators (such as leukotrienes and prostaglandins), and growth factors. These mediators recruit, differentiate, and activate monocytes and lymphocytes, augmenting both anti-inflammatory and anti-tumor immune responses.46
The release of anti-inflammatory factors, including lipid mediators, cytokines, and growth factors, helps terminate excessive inflammation, clear cellular and microbial debris, and initiate tissue repair and regeneration, thus restoring homeostasis. Consequently, acute inflammation plays a crucial immunostimulatory role during its early stages, and complete suppression of this response may impair antitumor immunity. Additionally, these factors prevent the overactivation of potentially damaging adaptive immune cells by stimulating regulatory T and B cells, which in turn modulate prolonged immune responses.47
Simultaneously, an oxidative burst associated with phagocytosis occurs, releasing toxic reactive species such as ROS (e.g., superoxide radicals and hydrogen peroxide). Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, a significant enzyme present in the plasma membrane and vascular system, activates leukocytes and releases reactive species like superoxide anions (O2•−) at the injury site, playing a key role in inflammation-induced peripheral vascular damage.48
Chronic Inflammation
Failure of the immune system to control or eliminate pro-inflammatory stimuli during acute inflammation can lead to chronic inflammation, which may result in tissue fibrosis, necrosis, autoimmune diseases, and cancer. Chronic inflammation is primarily characterized by the continuous release of inflammatory factors and persistent tissue damage.49,50 In this prolonged inflammatory state, macrophages and lymphocytes are the predominant infiltrating cells.51,52
Chronic inflammation is linked to various diseases, including diabetes, chronic heart failure, atherosclerosis, neurodegenerative diseases, and cancer, either directly or indirectly.53-56 In chronic inflammatory conditions, excessive ROS/RNS production contributes to oxidative stress and DNA damage, leading to the depletion of cellular antioxidants. The prolonged presence of inflammatory cells induces DNA damage, promoting cell transformation and increasing the mutation frequency.28 Additionally, chronic inflammation, through the continuous release of mediators and RNOS, activates pathways such as NF-κB and cyclooxygenase-2 (COX-2), which can induce mutations in proto-oncogenes and tumor suppressor genes, ultimately suppressing immune responses and promoting cellular malignancy.57 Studies have further demonstrated that unresolved long-standing inflammation is associated with increased carcinogenic potential and accelerated tumor progression.58,59
Relationship Between Oxidative Stress and Inflammation
Studies indicate that oxidative stress plays a pivotal role in the pathophysiology of inflammation.60,61 Numerous findings highlight the reciprocal relationship between chronic inflammation and oxidative stress, with each process enhancing the other. This mutual interaction creates a self-perpetuating cycle that promotes inflammatory cascades, chronic inflammatory disorders, and an increased risk of cancer.59,62,63
Chronic inflammation elevates reactive oxygen and nitrogen species (RONS) levels, contributing to oxidative stress. Conversely, oxidative stress, as a core component in chronic inflammation, activates various pro-inflammatory pathways, thereby advancing the pathogenesis of chronic diseases and cancer19,64. In response to inflammation or tissue damage, various intracellular and extracellular signals are released, which act as damage-associated molecular patterns (DAMPs), alarmins, or danger signals. These molecules initiate inflammatory cascades by engaging PRRs.65,66
The release of ROS into the extracellular environment can stimulate immune responses.67,68 Moreover, the oxidative stress state can lead to modifications of biomolecules (e.g., lipids and proteins), resulting in the formation of “structural neo-epitopes”. These neo-epitopes, or oxidation-specific epitopes (OSEs), serve as potent DAMPs recognized by receptors of the innate immune system.69,70 OSEs are now increasingly acknowledged as key mediators in inflammation and chronic diseases, appearing on oxidatively modified self-proteins, lipids, apoptotic cells, and cell debris, where they bind specific PRRs.67,69,71-74
Innate immune cells detect OSEs via specific receptors and initiate signaling pathways that activate humoral and cellular components of adaptive immunity to eliminate these modified molecules and prevent further inflammatory effects. However, if not adequately cleared, these OSEs act as DAMPs, potentially causing tissue damage, cell death, inflammation, and advancing lesions.75-77 During inflammation, an increase in oxygen uptake is accompanied by elevated ROS concentrations, promoting the recruitment and infiltration of mast cells and leukocytes into injury sites and enhancing RONS formation. This process, termed the “respiratory burst”, becomes more pronounced in chronic inflammation, heightening the risk of chronic diseases and cancer.17,78-82
ROS regulate inflammation by initiating pro-inflammatory signaling cascades and activating cytokines, chemokines, and the NLRP3 inflammasome.83,84 Notably, mitochondria-derived ROS can activate transcription factors such as NF-κB, signal transducer and activator of transcription 3 (STAT3), activator protein 1 (AP-1), hypoxia-inducible factor-1 alpha (HIF-1α), nuclear factor of activated T cells (NFAT), and NFE2-related factor 2 (Nrf2), all of which mediate cellular stress responses.19,84,85 These transcription factors are critical in regulating cellular processes and gene expression in response to stimuli, including cytokines, growth factors, stress, inflammation, and infections.86
The cytosol can produce reactive species from multiple endogenous and exogenous sources. Cytosolic ROS, especially superoxide and hydroperoxides, are often generated in mitochondria and redoxosomes, from which they diffuse into the nucleoplasm, where they interact with nucleic acids and other nuclear components 87. In the cytosol and redoxosomes, pro-inflammatory enzymes such as NADPH-oxidases (NOX), inducible nitric oxide synthase (iNOS), COX2, 5-lipoxygenase (5-LOX), and heme-oxygenase-1 (HO-1) can amplify ROS production.87,88
Additionally, ROS can induce pro-inflammatory mediators, such as COX2, HIF-1α, and iNOS, which stimulate the expression of inflammatory cytokines (e.g., interleukin [IL]-1, IL-6, and tumor necrosis factor [TNF]) and chemokines (e.g., IL-8 or CXCL8), all of which are implicated in the pathogenesis of chronic diseases, oxidative stress-related inflammation, and cancer.87,88 These mediators can also trigger epigenetic changes in specific miRNA profiles, which are involved in the initiation and progression of inflammation-related tumors.89,90 Moreover, cytokines and chemokines activate NF-κB and STAT3, both of which play crucial roles in cancer progression.91-93 Research has demonstrated that ROS-induced COX-2 and NF-κB can increase oncogenic K-Ras levels, linking chronic inflammation to cancer progression.94 Mutations in Ras isoforms, especially K-Ras, which occur in approximately 25% of all cancers, promote cell proliferation, tumor growth, and angiogenesis.95-97 During inflammation, Ras stimulates the expression of various inflammatory mediators, including pro-inflammatory cytokines (e.g., IL-1, IL-6, and IL-11) and the chemokine IL-8.98 IL-1, IL-6, and TNF-α promote cancer cell growth and tumorigenesis through ROS generation and DNA damage.99-102 This persistent inflammatory and oxidative environment establishes a vicious cycle that can damage neighboring healthy epithelial and stromal cells, leading to carcinogenesis over time.87,103
Inflammation in Cancer
The association between inflammation and cancer risk was first recognized in the late 19th century by Rudolph Virchow.104 Research has demonstrated that inflammation can either promote cancer progression or inhibit it, depending on the type (acute or chronic), extent (local or systemic), and timing (before or after cancer onset) of the inflammatory response.105 Acute, or therapeutic, inflammation can induce cancer cell death by activating effective anti-tumor immune responses 106-108. Conversely, chronic or unresolved inflammation fosters an immunosuppressive environment conducive to tumorigenesis, metastasis, and therapeutic resistance.109,110 High levels of tumor-infiltrating lymphocytes (TILs) in localized inflammation correlate with improved prognosis due to enhanced anti-tumor immunity.111,112 In contrast, systemic inflammation often promotes tumor growth, worsens prognosis, and is associated with the progression of various cancers, such as colorectal cancer (CRC).113-115 TILs, frontline soldiers of the adaptive immune system, are recruited to the tumor microenvironment (TME) to fight against tumor cells.116 Systemic inflammation can support tumor growth and spread by increasing vascular permeability, aiding cancer cell movement through blood and lymph vessels, and enhancing endothelial adhesion in metastatic environments.117 The timing of inflammation affects the occurrence of cancer. Inflammation occurring before cancer onset may promote tumor development, whereas inflammation occurring post-cancer may hinder its progression.105
The differential effects of inflammation types are primarily due to variations in immune cell activity and molecular interactions. For instance, acute inflammation, stimulated by recombinant cytokines, TLR activators, and chemotherapeutic agents, enhances TIL infiltration, M1 macrophage polarization, and NK cell activity within the TME, subsequently boosting adaptive immunity, inhibiting tumor growth, and improving treatment efficacy.58,118-120
In contrast, chronic inflammation consistently supports cancer development, regardless of its timing. Cancer-promoting inflammation often results in increased immune cells, such as myeloid-derived suppressor cells (MDSCs),121 regulatory T cells (Tregs),122 tumor-associated fibroblasts, and macrophages.123 These cells inhibit cytotoxic T lymphocyte (CTL) responses, weaken immune surveillance, and enhance immunosuppressive activity within the TME. Chronic inflammation also activates the NF-κB pathway, mainly through elevated TNF-α levels, which supports tumor cell survival and growth.124-126 TNF-α interacts with TNFR1 and TNFR2 receptors, activating pathways like c-Jun N-terminal kinase (JNK), NF-κB, and caspase cascades in cancer cells, with effects that may vary based on dose and receptor expression.127-130
Evidence suggests that unresolved chronic inflammation significantly increases the risk of cancer as persistent inflammation contributes to malignancies across various cancer types.99,131,132 Cancer-related inflammation (CRI) is now regarded as the seventh hallmark of cancer as it links chronic inflammation to cancer via enhanced proliferation and survival signals, facilitating angiogenesis, invasion, and metastasis.99,133-135 This connection is influenced by two pathways: intrinsic and extrinsic. The intrinsic pathway fosters oncogenic transformation, initiating inflammatory cascades, while the extrinsic pathway, mediated by tumor-infiltrating leukocytes, primarily M2 macrophages, regulates CRI. Both pathways recruit transcription and tumorigenic factors such as NF-κB, STAT3, and HIF-1, crucial for promoting tumor-associated inflammation and suppressing anti-tumor immune responses (Figure 1).136-138
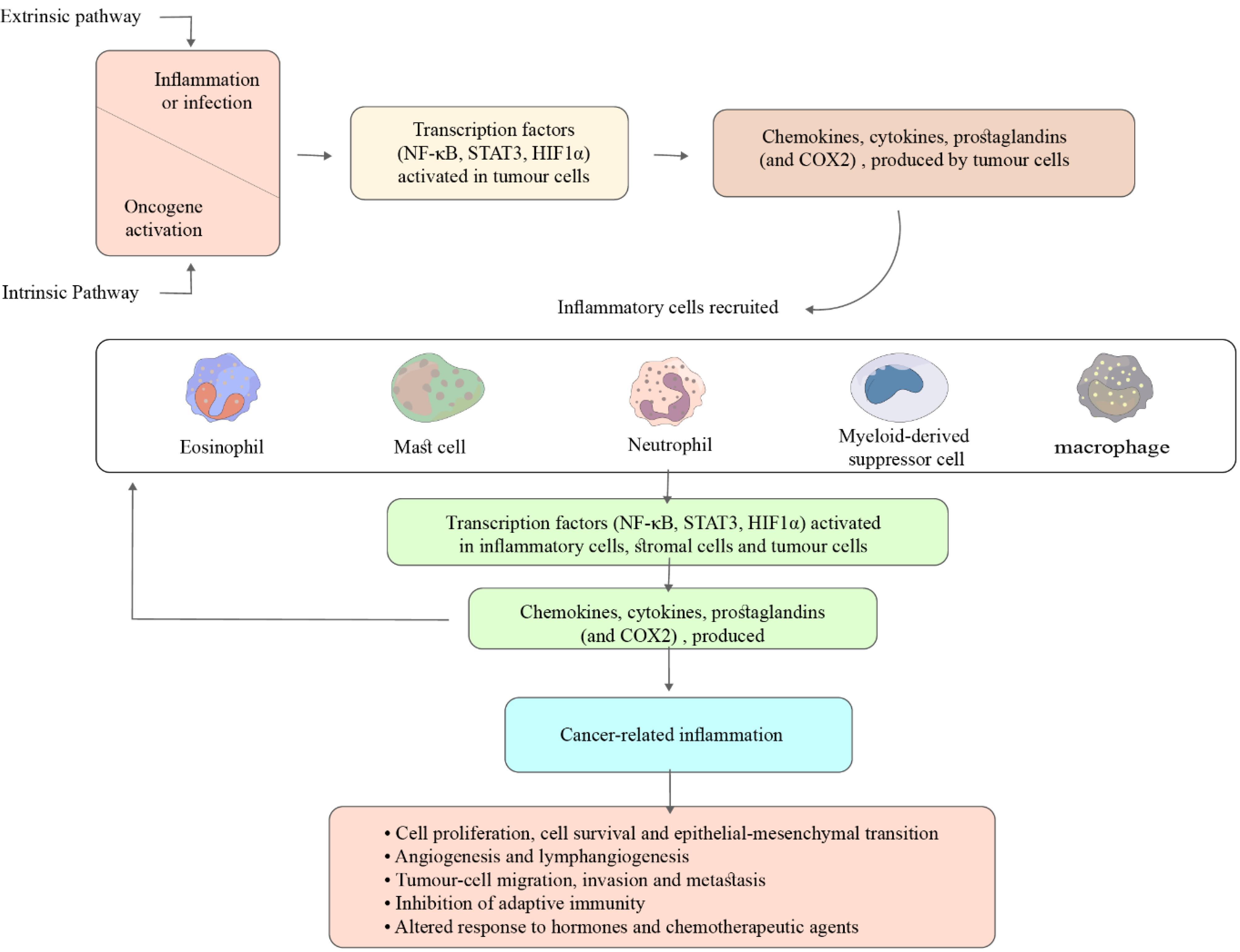
Figure 1.
Two Interrelated Pathways linking Inflammation and Cancer. Activation of both paths leads to an increase in cancer cell proliferation, angiogenesis, and metastasis
.
Two Interrelated Pathways linking Inflammation and Cancer. Activation of both paths leads to an increase in cancer cell proliferation, angiogenesis, and metastasis
A hallmark of CRI is the infiltration of leukocytes, particularly tumor-associated macrophages (TAMs), from blood vessels into affected tissues via chemotaxis.139 This is accompanied by elevated levels of pro-inflammatory cytokines, such as IL-6, IL-1β, NF-κB, and TNF-α, and chemokines like CCL2 and CXCL8. These chemokines promote inflammation and tumorigenesis by creating an inflammatory microenvironment that contributes to cancer development.140-143 The NF-κB pathway plays a central role in regulating inflammatory responses. Inflammatory cytokines, chemokines, and growth factors promote the production of transcription factors, including STAT3, HIF-1, AP-1, and heat shock factor-1 (HSF1), which can affect cancer-specific microRNA expression.19 It is widely recognized that reactive species and oxidative stress, as pathophysiological events, can induce chronic diseases and cancer via multiple mechanisms.19,144 They may drive cancer progression through initiation, promotion, and progression stages, ultimately leading to tumor cell migration or metastasis. Furthermore, chronic inflammation is a primary mediator of cancer induction, influenced strongly by reactive species.48
The roles of chronic inflammatory mediators in cancer are complex and sometimes paradoxical.105,110 While uncontrolled inflammation increases cancer risk by promoting proliferation, angiogenesis, invasion, and metastasis, certain inflammatory mediators, such as lipid messengers (prostaglandins) and polypeptide messengers (cytokines and chemokines), can destroy cancer cells and suppress tumor progression by activating effector and cytotoxic immune cells.105,110 Therapeutic strategies targeting CRI aim to reduce cancer incidence and progression. CRI-based approaches may help shift the tumor-supporting inflammatory environment toward a tumor-suppressive microenvironment, enhance anti-tumor immune responses, and prevent cancer development.
Oxidative Stress in Cancer
The TME, a complex milieu, comprises diverse types of intracellular and extracellular components, inflammatory agents, and immune cells that collectively influence cancer cell behavior toward increased vascularization and metastasis by creating an immunosuppressive environment.145,146 The physical conditions and cellular makeup of the TME shape the behavior of cancer cells and modify the immune response landscape.145 Within the TME, immunosuppressive cells, including type II macrophages (M2), MDSCs, regulatory T cells (Tregs), tumor-infiltrating dendritic cells (TIDCs), and reactive species, proliferate. These components hinder the function of immune effector cells, such as natural killer (NK) cells and cytotoxic CD8 + T cells (CTLs), thereby enabling tumor cells to evade immune surveillance and promoting tumor progression.147-149 Additionally, tumor cells exhibit elevated levels of ROS. Excessive or prolonged ROS production can lead to tissue destruction, oxidative stress, and potentially higher cancer risk.82,150
Oxidative stress-induced signaling pathways influence multiple facets of cancer cell behavior, including cell cycle regulation, proliferation, apoptosis, energy metabolism, morphology, adhesion, motility, and angiogenesis.6,151,152
Cancer cells exhibit altered metabolism to meet their increased energy demands for rapid growth and proliferation.20 To support cellular functions such as signal transduction and gene expression, cancer cells generate more ROS than normal cells.20 Accumulated ROS in mitochondria fosters oxidative stress and tumorigenesis through mitochondrial DNA (mtDNA) mutations and activation of oncogenic signaling pathways.153,154 Oxidative stress also contributes to the initiation and progression of cancer by inducing chromosomal abnormalities and oncogene activation 155, further associating with cancer angiogenesis, invasion, and metastasis.156,157 Extensive research has demonstrated that oxidative stress directly affects various cancer types, including colon,158 colorectal,159 hepatocellular,160 ovarian,161 breast,162 and brain163 cancers.
An overabundance of RONS is critical in regulating cancer stem cells and tumor behavior through direct DNA damage or formation of N-nitroso compounds.164 Regulation of oxidative stress levels is essential for both cancer progression and anti-cancer immunity. ROS can play dual roles in carcinogenesis, depending on their levels. High ROS levels may promote tumor initiation and progression by inducing genetic mutations, activating oncogenic factors, and inhibiting tumor suppressors, such as Kirsten rat sarcoma viral oncogene homolog (KRAS) and p53, thereby fostering cancer initiation.161,165 On the one hand, high levels of ROS can initiate tumorigenesis and cancer progression.166,167 Excessive ROS accumulation also enhances tumor growth by disrupting anti-tumor immune cell function, such as T cells and NK cells, and promoting M2 macrophage polarization within the TME, further supporting tumor progression.168-171 Conversely, extreme accumulation of ROS can exert anti-oncogenic effects by inhibiting tumor cell proliferation. Excessive ROS can induce cancer cell apoptosis through the activation of endoplasmic reticulum stress-, mitochondrial-, and p53-mediated apoptotic pathways, cell cycle arrest, and the ferroptosis pathway in cancer.148,161,172-174
Inflammation and stress-associated signaling pathways play pivotal roles in the development of cancers.19,175 It has been demonstrated that endogenous ROS-induced oxidation acts as a physiological secondary messenger in cell signaling and apoptosis and modulates or modifies multiple transduction pathways.176-178 The MAPK, phosphoinositide-3-kinase- (PI3K-) Akt, Janus kinases/signal transducers, and activators of transcription (JAK/STAT), (ERK/MAPK), Ca signaling, the activator protein (AP-1), Keap1-Nrf2-ARE, and mPTP are intracellular pathways which are regulated by ROS.178-180
NF-𝜅B, epidermal growth factor (EGF), hypoxia, tumor necrosis factor-α (TNF-α), IL-1, IL-6, TNF-α, and interleukin-1β (IL-1β) have long been considered as pro-inflammatory signaling pathways, and mitochondria-derived ROS induce the upregulation of these stimuli in a concentration-dependent manner.83,178,181 These pathways play an important role in cancer development.182,183
ROS-induced intracellular pathways are also partially mediated by MAPKs that are involved in cancer.179,184 PI3K/AKT and RAS/MEK/ERK (ERK/MAPK) pathways are two major signaling pathways in cancer which enhance tumor cell growth, survival, and metabolism of cancer cells and have been identified as promising therapeutic targets for cancer therapy.185-188 Inhibiting any of these pathways can prevent cancer progression and improve anti-cancer therapy.185,187,189
Although many studies have shown that genetic mutations can dysregulate kinase activity and hyperactivate the MAPK pathway during induction and progression of tumorigenesis and promote the growth of tumor cells,182,190 other studies indicated tumor suppressive activity of this pathway through induction of senescence and ubiquitination and degradation of participated essential proteins in cell cycle and survival.191 Senescence is a stable and terminal state of cell cycle arrest associated with changes in different macromolecules and overproduction of proteins (cytokines, proteases, and growth factors), collectively termed the senescence-associated secretory phenotype (SASP). The entry of cells into senescence by proliferative arrest acts as a barrier against tumorigenesis, which could be a desirable outcome for any anticancer therapy.192,193 It should be noted that SASP possesses anti-tumorigenic and pro-tumorigenic potential.194
MAPK/ERK pathway activated by RAS promotes degradation of proteins necessary for both cell migration and progression through the cell cycle.191 However, due to the prominent role of ROS-dependent MAPK signaling in cancer development, blocking this pathway could be effective in reducing cancer growth and increasing the effectiveness of anticancer therapies.195
It can be concluded that the MAPK/ERK pathway shows both oncogenic/tumor suppressor activities depending on the strength of its activity and protein degradation related to senescence and tissue-specific TME191,196. It seems that ROS can stimulate, inhibit, or regulate tumorigenesis through monitoring the activation of MAPK pathways in different stages of various cancers; therefore, they can be highly promising therapeutic targets for cancer therapy. 197
Immune Responses in Inflammation, Oxidative Stress, and Cancer
The responses of the immune system are activated against tumor cells via different processes, including innate/adaptive immune responses.198,199 The innate immune response with neutrophils, monocytes, and DCs and the adaptive immune response with B and T lymphocytes are orchestrated in tumor response. The first line of immunity against invading cancer or pathogens is the activation of innate immunity.198 This primary response stops the initial spreading of infection and does not activate adaptive immunity and more specific defense mechanisms.200 In fact, through inflammation, adaptive and innate immune responses tend to eradicate invasion. Unfortunately, these inflammatory responses can be detrimental and probably affect different stages of cancer development.143 Results of several studies indicate the favorable role of the inflammatory condition in the growth of tumors.143,201 It is well accepted that acute inflammation is an inevitable part of the anti-pathogenic response and tissue repair.202,203 Interaction between host immune responses and cancer cells in solid and hematological cancers is complex and inevitable.204,205 DCs are professional APCs, the main function of which is to capture antigens and present them to effector memory T cells (T EM cells) and stimulate effective anti-tumor immune responses.206 The substantial roles of NK and lymphocytes in immune surveillance have been proven for a long time. Different mechanisms, such as induction of the production of interferons (INFS) and other mediators indirectly stimulate durable antitumor immunity.207,208
Innate Immunity
At the beginning of inflammation, innate immune cells are the first line of the immune defense against inflammation.209 Various biological components such as lymphocytes and macrophages are involved in the initiation and propagation of the inflammation.51,52 This process is facilitated by the overexpression of cytokines, chemokines, and growth factors. These mentioned cells contribute to the enhancement of defense responses that counteract the inflammation-induced agents.210,211 The inflammatory process is directly related to neutrophil recruitment and clustering.181 Neutrophils are required for selective IL-1β induction, and accumulation of ROS modulates IL-1β-derived neutrophil clustering. Inflammatory-stimulated neutrophils are among the essential sources of ROS production. During pathogenic infections, neutrophils upregulate IL-1β secretion in a ROS-dependent alternative pathway. IL-1β plays a determinative role in establishing inflammatory responses.212,213
The main partners of phagocyte cell systems, neutrophils and macrophages, have been identified as the first immunological line of host defense against various invading microorganisms during different infections, CRI, exercise, and phagocytosis of cancer cells.214,215 Based on the stimulation of effector agents, neutrophils are functionally classified into two N1/N2 subdivisions that exhibit anti/pro-tumorigenic activities. They, directly/indirectly by the production of different mediators such as TNF-a, NO, and H2O2 or cytokines such as GM-CSF and IFN-g are involved in immunosuppressive and immune stimulatory responses, respectively.216,217 Neutrophil cells were considered as the first responders to acute inflammation, whereas monocyte/macrophages were recognized as key cellular components involved in the development of chronic inflammation.218,219 However, several studies indicated that in chronic inflammation, neutrophils participate in the induction of inflammation and release inflammatory mediators such as cytokines, highlighting the recruitment of monocytes/macrophages in acute inflammatory responses. They exhibit dual roles in the promotion or maintenance of many diseases. The plasticity of macrophages and neutrophils allows them to alter their phenotype and various functions in response to different stimuli and environments.220-223
Many studies have proved the role of oxidative stress, especially ROS, in the disturbance of functional polarization of macrophages.224,225 Thus, in this section, we discussed macrophage reprogramming and how ROS drives macrophage polarization.
Macrophages, as innate immune cells, are distributed throughout the body’s tissues and direct innate and adaptive immune responses by the production of biological/pathological active molecules. Hence, macrophages play crucial roles in maintaining tissue homeostasis or promoting malignancy. Macrophages can be differentiated into various types in different conditions.226,227 Physiological and pathological processes have a decisive role in macrophage reprogramming. An imbalance of macrophage M1-M2 polarization is often associated with various diseases or inflammatory conditions, including obesity, cancer, and rheumatoid arthritis. In addition to the structural and functional differences between the two macrophage phenotypes, the metabolic axis of macrophages in amino acid arginine metabolism. The ability to generate high levels of pro-inflammatory mediators such as IFN-γ, IL -12, IL-1𝛽, TNF-𝛼, and superoxide anions is a hallmark of classically activated macrophages (M1) that gives them the full capability to kill tumor cells.228-230 M1 macrophages, by induction of nitric oxide synthase enzyme, produce nitric oxide (NO) that plays a vital role in killing microbial components and tumor cells and eradicating tissue debris or microbial residues that neutrophils have failed to remove. Additionally, NO contributes to the clearance of apoptotic bodies of dead neutrophils. Therefore, M1 macrophages are involved in the initial stages of the inflammation process.231,232 In contrast, the function of alternatively activated M2 macrophages is distinct from the classical phenotype. M2 macrophages release high levels of anti-inflammatory cytokine mediators, including IL-4, IL-10, and tumor growth factor β1 (TGF-β1). Furthermore, low levels of IL-12 expression induce production by the arginase enzyme. Arginase plays a role in reviving inflamed and demolished extracellular matrix (ECM) by stimulating the production of proline and polyamines. The functionality of macrophages may alter from pro-inflammatory to anti-inflammatory phenotype, which can contribute to healing by removing tissue debris.233
Neutrophils are the first line of defense against inflammation, infection, and cancer. They constitute 50%-70% of the total circulating white blood cells in humans. An elevated level of circulating neutrophils may be a prognostic indicator for cancer. The TME releases neutrophils in the bone marrow and bloodstream by secretion of granulocyte colony-stimulating factor (G-CSF).220,234,235 Neutrophil infiltration is significantly increased during CRI, the seventh hallmark of cancer. The initial phase of inflammation is correlated with local recruitment and the rapid influx of neutrophils into the site of inflammation. They produce pro-inflammatory cytokines/chemokines that make up neutrophil extracellular traps (NETs), which can destroy most types of microbes and affect the pathogenesis of many diseases such as cancer and autoimmune disorders. They use both pro/anti-inflammatory signals to stabilize immune responses.236 NF- κB activation is stimulated following the identification of PAMPs via TLRs and other PRRs that cause neutrophil production. Accumulation of neutrophils initiating MAPK signal transduction pathways that induce sequential activation of Ras, Raf-1, mitogen/extracellular signal-regulated kinase (MEK), and the extracellularly regulated kinase (ERK) pathways, which result in the generation of pro-inflammatory cytokines, including IL-8 and macrophage inflammatory protein-1a (MIP-1a) that induce pro-cancer factors like TGF-β.237-240 Neutrophils, as potent leukocytes in host immunity, are considered a vital link between innate and adaptive immune responses. Neutrophils aim to boost the efficacy of monoclonal antibodies-related treatments and increase the cytotoxic activity of cancer therapy methods in cancer patients. The early evidence demonstrated that human neutrophils mediate the production of many new antimicrobial proteins in response to various stimuli and synthesize a high level of ROS via the myeloperoxidase (MPO) and NADPH oxidase enzymes.241,242 ROS are volatile drivers that act as signaling molecules and cause damage to many biological components of microbes and the host.243 ROS-related neutrophils regulate both cytokine secretion and apoptosis process, playing an essential role in balancing inflammation.244 Therefore, neutrophils/macrophage-targeting agents offer new approaches for clinical translation.
Tumor-associated macrophages and neutrophils (TAM and TAN) are primary inflammatory cells and potent immunosuppressive components of tumors that promote tumor induction and metastasis.236,245 In the last decade, the role of neutrophils and macrophages in cancer has been extensively investigated. Several studies showed that the anti/pro-tumoral activities exerted by neutrophils and macrophages are controlled mainly by the tumor niche. In the inflammatory media, particularly the tumor site, severe deficiency of adequate oxygen (hypoxia) by inducing the production of type 1 and type 2 hypoxia-inducible factors (HIFs) and NF-𝜅B affects the polarization, killing potency, secretome, penetration, and adhesion to endothelial cells, which are often administrated by tumor-infiltrated neutrophils and macrophages and support tumor formation, advancement, and other cancer-related processes (metastasis, angiogenesis, etc). Tumor-associated macrophages, via diverse mechanisms, can initiate chronic inflammation and get involved.246,247
In a tumor environment, macrophages and neutrophils are mainly related to cancer development. Tumor-associated neutrophils (TANs) suppress anti-tumor immune responses by generating a high rate of angiogenic-associated factors such as matrix metallopeptidase-9 (MMP-9), IL-10, vascular endothelial growth factor (VEGF), and reactive oxygen/nitrogen species.248,249 However, the ROS released by neutrophils can cause DNA damage and mutations,250,251 which are essential for cancer initiation, cell proliferation, CRI, and immune suppression.252 Compared to healthy cells, higher levels of neutrophil-derived ROS, including superoxide anion and hydrogen peroxide, are found in tumor cells that provoke pro-inflammatory and pro-tumorigenic activities and excite oxidative stress that eventually leads to cancer induction and progression.
In promoting carcinogenesis and tumor progression (Figure 2), there are many functional similarities between neutrophils and macrophages. Interactions between them and other tumor-infiltrated-immune cells are influenced by oxygen-sensing pathways, which may lead to pre-malignancy transformation. Oxygen-sensing pathways can modulate neutrophil function and survival responses. However, the exact mechanisms of neutrophils and other immune cell activities are not yet understood due to their highly plastic nature and multilateral functions in cancer. Thus, there are no unified and valid data on the function of neutrophils and immune cells in all diseases and cancers.
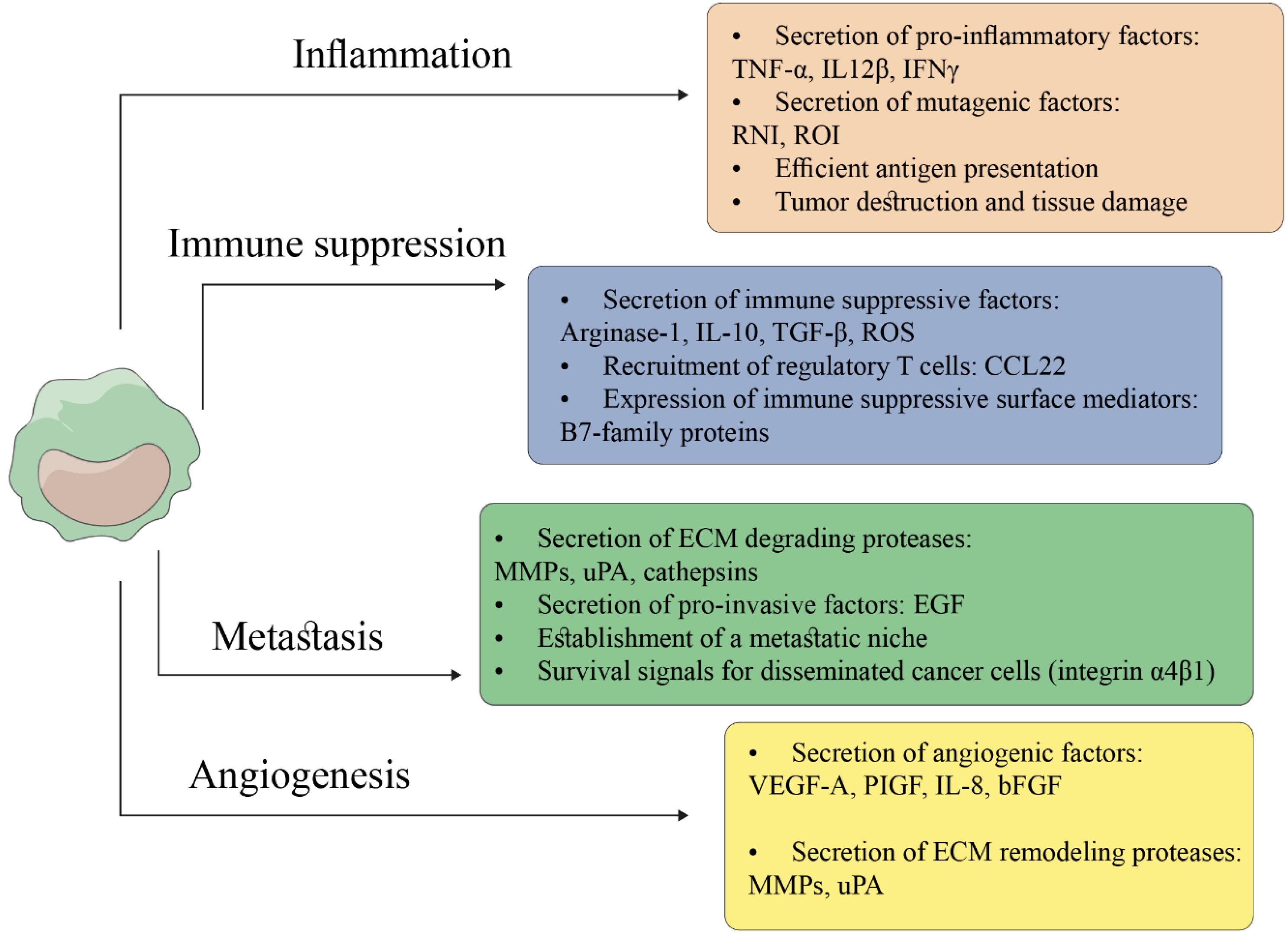

Figure 2.
Immune Suppressive, Tumor Promotion Activities of TAMs in the Tumor Milieu. TAMs play important roles in tumorigenesis and tumor progression. (a) TAMs, by inducing the production of pro-inflammatory and mutagenic factors, are heavily implicated in cancer-related inflammation. (b) Mediators and enzymes secreted by immune-suppressive TAMs via dysfunction of conventional activity of cytotoxic T cells suppress the anti-tumor immune response, leading to infiltration of regulatory T cells. (c) TAMs, by secretion of angiogenic growth factors, promote the formation of new more permeable tumor vessels and production of tissue remodeling proteases (e.g., MMPs). (d) TAMs, by digestion of ECM components through proteolytic enzymes, particularly matrix metalloproteinase (MMPs), and secretion of invasion-inducing growth factors, facilitate the movement and migration ability of malignant cells to invade surrounding tissues. TAM: tumor-associated macrophages; ROI: reactive oxygen intermediates; RNI: reactive nitrogen intermediates; ECM: extracellular matrix
.
Immune Suppressive, Tumor Promotion Activities of TAMs in the Tumor Milieu. TAMs play important roles in tumorigenesis and tumor progression. (a) TAMs, by inducing the production of pro-inflammatory and mutagenic factors, are heavily implicated in cancer-related inflammation. (b) Mediators and enzymes secreted by immune-suppressive TAMs via dysfunction of conventional activity of cytotoxic T cells suppress the anti-tumor immune response, leading to infiltration of regulatory T cells. (c) TAMs, by secretion of angiogenic growth factors, promote the formation of new more permeable tumor vessels and production of tissue remodeling proteases (e.g., MMPs). (d) TAMs, by digestion of ECM components through proteolytic enzymes, particularly matrix metalloproteinase (MMPs), and secretion of invasion-inducing growth factors, facilitate the movement and migration ability of malignant cells to invade surrounding tissues. TAM: tumor-associated macrophages; ROI: reactive oxygen intermediates; RNI: reactive nitrogen intermediates; ECM: extracellular matrix
Adaptive Immunity
Environmental exposures such as inflammation can also influence cancer incidence through several mechanisms.253 Although the role of inflammation is very prominent in innate immune responses, its importance has been proven in the initiation and development of adaptive immune responses. Components of adaptive immunity, such as lymphocytes, cooperate with innate immune cells and get involved during acute and chronic inflammation via the generation of effector/memory cells and organize the inflammatory responses.254
In local inflammatory and acute-phase responses, non-specific innate immunity may be enough to control an inflammatory disease. Otherwise, in uncontrollable/chronic inflammation, specific immune response (especially DC and T-cell immunity) occurs in specialized lymphoid tissue.255,256 The lymph nodes and spleen act as a screening system, facilitating the transmission of lymphocytes and antigens through the tissue. The antigen can be loaded by macrophages and DCs in the secondary lymphoid tissue, and the filtered lymphocytes can interact with antigen-bearing DCs or macrophages. Therefore, they differentiate into effector/memory cells to eliminate antigens.257,258
According to the T cell receptor (TCR) expression, T-cells are divided into two major types: γδ and αβ. Different effector functions of αβ cells are classified into: (1) naïve CD4 + T helper (Th) cells (that coordinate the generation of other effector cells), which mediate tolerance and consist of Th1 cells (that produce IFN-g and IL-12), Th2 cells (that produce IL-4, IL-5, and IL-13), Th17 cells (that produce IL-17A, IL-17F, and IL-22f), T regulatory (Treg) cells, and natural killer T (NKT) cells; and (2) cytotoxic CD8 + cells (CTLs) that mediate clearance and suppressing antigen-specific inflammation, killing tumor-intracellular pathogens, thereby contributing to long-term protection.259,260 Interestingly, T cells display tumor suppressive and tumor promoting characteristics (beneficial and harmful activities, respectively). There is also evidence that many of the T cell subpopulations, which are found in solid tumors, participate in tumor initiation, progression, and metastasis. Elevated numbers of T cells, specifically activated Th cells and CTLs, are associated with improving the survival rates in different cancers.261
There are significant differences between non-T cell-inflamed tumors and T cell-inflamed tumors.262 T cell-inflamed tumors are accompanied by the expression and activation of type I IFN (α, β), and production of particular chemokines that attract T cells, antigen presenting cells, cytotoxic effector molecules, and CD8 + T cells with a dysfunctional phenotype. High expression levels of PD-L1, indoleamine-2, 3-dioxygenase (IDO), and FOXP3 + regulatory T cells (Tregs) can induce IFNs inhibitors. In other words, when CTLs are affected by immunosuppressive mediators in the TME and peripheral blood, they lose their antitumor function. Exhausted T cells are characterized by an increased expression of inhibitory markers and a progressive and hierarchical loss of function. However, it seems that the use of immunological adjuvant reverses suppressor activity of T cell exhaustion and accelerates host immunity, which can open up new horizons for improving outcomes in severe inflammatory disorders.262 In contrast, in the non-T cell-inflamed phenotype, the expression of type I IFN, CD8 + T cells, and IFN-inducible inhibitory factors does not occur.262-265
The Effect of Inflammation and Oxidative Stress on Pro-inflammatory Cytokines
Activation and interaction of various effectors and transcription factors, such as STATS, NF-κB, AP-1, SMAD, cytokines, chemokines, caspases, and so on, with each other and their environment can regulate a broad spectrum of cellular and molecular functions within tumors.266 Pro-inflammatory cytokines and chemokines are small second messenger molecules that are secreted during stress or in the damaged tissues. They synchronize diverse biological activities such as cell differentiation, proliferation, and cancer progression by forming a cytokine network within the TME.99,267
Regardless of their sources and receptors, they display various roles in the immune and inflammatory milieu to either induce tumoricidal function or promote tumor progression.266 It is evident that cytokines have the susceptibility to modulate the redox status of T cells, based on position, stage, and severity of the disease, skewing T cell differentiation toward Th1, Th2, Th9, Th17, Th22, and Treg subsets and inducing anti-tumor immunity, pro-tumorigenic effects and homeostasis maintenance in various types of cancer268 (Figure 3). In different conditions and environments, cytokines augment T-cell differentiation toward specific cell subtypes by the overexpressing of particular growth factors, thus attenuating differentiation of the other T-cell subpopulation. Differentiation towards the Th1 system leads to the production of pro-inflammatory cytokines such as IL-2, IL-12, INF-γ, and TNFα and T-cell-mediated immune responses (cellular immunity).269,270 Conversely, differentiating towards the Th2 system develops the generation of anti-inflammatory cytokines, including IL-4, IL-10, IL-13, and antibody-mediated immune responses (humoral immunity).271,272 However, some of them, such as IL-2 and IL-6, often exhibit pleiotropic properties and act as proliferative factors of immune cells in systemic acute phase responses.273-275
Figure 3.
Cytokines and Transcription Factors Involved in Differentiation of T Cell Subsets from Naïve T Cells
.
Cytokines and Transcription Factors Involved in Differentiation of T Cell Subsets from Naïve T Cells
The uncontrolled inflammatory cascade results from the interaction between oxidative stress and pro-inflammatory cytokines, especially TNF-α and NF-κB, which are two major transcription factors responsible for pro-inflammatory gene expression. Cytokines are involved in both local and systemic cancer-related inflammatory responses via interaction and cross-talk with inflammatory mediators and oxidative stress.276
It seems that cytokines and oxidative stress are considered as major stimuli for the activation of intracellular signal pathways and augmentation of the inflammatory cascade, which lead to local damage, edema, inflammation, epigenetic modulation, and/or cell death.277-279 The intracellular signaling pathways activated by mentioned performers, in turn, activate inflammatory-related processes such as the expression of protein kinases activated by mitogens (MAPK). Oxidizing agents by releasing superoxide and nitric oxide (oxidants) enhance the expression of nuclear transcription factor-κB, signal transducer activator of transcription-3 (STAT3), and production of primary inflammatory mediators and cytokines, particularly iNOS, TNF-α, IL-1β, IL-8, IL-6, and IL-12 that customarily originate from activated leukocytes and have critical roles in initiation and amplification of inflammatory cascade. In the inflamed environment, infiltrated and circulating leukocytes adhere to the endothelium via adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1) and induce increased serum levels of stress-associated cytokines and chemokines by attaching to the inflammatory mediators.280,281
Oxidative stress increases the production of cytokines via various mechanisms, including up-regulation of growth factors, transcription factors such as NF-κB, ECM elements, and AP-1.282,283 TNF-α and IL-1β synergically participate in strengthening the inflammatory responses. Activated macrophages stimulate TNF-α secretion in tumoral and inflamed parts. TNF-α induces ERK1/2, JNK, p38, and NF-κB gene expression through divergent procedures, which results in signaling cell death and microcirculatory circumstances that occur during stages of inflammation.284,285 Increased inflammatory cytokines and chemokines, growth factors, cell surface adhesion molecules, prostaglandin synthases, and oxidant synthase are mechanisms mediated by NF-κB overexpression. Numerous studies indicated that during CRI, NF-κB creates genetic alterations (amplification, mutations, or deletions) in cancer cells.286-288 Additionally, expression of NF-κB is induced via the inflammatory cytokines of TNF-α and IL-1b, as well as TLR-MyD88 pathway, the identifier of pathogen-associated conserved molecular structures (MAMPs or PAMPs). Moreover, NF-κB activation plays a critical role in the hypoxic response, through HIF-1α.289,290 Several experimental pieces of evidence showed the suppressive activity of oxidative stress on the expression of IFN-g and IL-17A originated from differentiated Th1 cells and Th17, respectively, boosting IL-4 expression in inflammatory-related disorders by inducing ERK1/2 activity. On the contrary, IL-12 and IFN-g signals lead to the amplification of Th1 responses and undermine Th2 development by repressing the IL-4 gene.
Inflammasomes, as a complex of cytosolic molecular platforms and innate immune system receptors of the tissue injury-released exogenous PAMPs, and endogenous danger signals or DAMPs possess an upstream sensor protein of the NOD-like receptor (NLR) family, the adaptor protein ASC, and the downstream effector caspases that induce maturity of inflammatory cytokines such as IL-1β, IL-18, and activation of caspases, particularly caspase-1 in stress status, inflammatory disorders, and oncogenic viral infections.291,292 Activation of an inflammation-related caspase (caspase-1) triggers the release of pro-inflammatory cytokines and results in a particular type of pro-inflammatory programmed host cell death known as pyroptosis, which is different from apoptosis.293
Many contradictions are reported regarding the role of the ROS-dependent pathway in the stimulation of forming inflammasome complexes such as NLRP3 inflammasome activation.63,294 However, several studies described the activator/regulatory role of mitochondrial ROS (mtROS), NADPH oxidase-derived ROS, and other mediators of ROS as key inflammasome activating signals. An elevated level of ROS, by NLRP3 inflammasome stimulators, upregulates the expression of transcriptional factors, including transcription factor NF-κB and AP-1 through MAPKs, which increases the secretion of pro-inflammatory cytokines.295-297
It was demonstrated that the pattern of the cytokines could be modified by the oxidative microenvironment, which might influence immune pathologies.
In cancer conditions, the way inflammation participates in the protection/destruction of body tissues is an important and challenging issue.
Although oxidative stress is involved in various age-related conditions and is harmful to human health, it can also have benefits as a therapeutic approach and can become toxic to cancer cells.12 Balancing between pro-oxidants and anti-oxidants should be performed with great sensitivity because their conditions differ in various cancers and chronic inflammatory diseases.298 Understanding these contradictions is very important. Evaluating the communication between oxidative stress, inflammation, and pathogenicity can be a precious tool for discovering targeted preventive, therapeutic, and diagnostic strategies in humans. Identifying and directly assessing the expression levels of stimulatory agents, assessing the amounts of biological damage caused by oxidants and inflammatory agents, and assessing anti-oxidant conditions such as their activities, levels, and capacity promise alternative approaches to describe their critical roles in carcinogenesis and cancer development in clinical samples of cancer patients.
Inflammation and oxidative stress have been proposed as promising and efficient anti-cancer approaches.90,299,300 Depending on dosage and amounts, ROS may initiate or inhibit apoptosis and skew cellular and molecular pathways toward necrosis by stimulating c-Jun N-terminal kinase (JNK) and caspases. Several studies have already reported that the toxic threshold level of ROS induces cancer cell death by stimulating biological responses in chronic inflammatory states. Autophagy, apoptosis, adaptation, and enhanced drug sensitivity are mechanisms that can be mediated by increased levels of ROS.301,302
Immunomodulators
As previously stated, oxidative stress with inappropriate activation of the immune cells may exacerbate inflammation and cause many inflammatory and infectious diseases such as cancer. Cancer cells have higher levels of ROS compared to normal cells. Persistent high levels of ROS can result from an imbalance between oxidant production and antioxidant responses.303 This perturbation is usually associated with changes in ROS-dependent metabolites that contribute to the progression of inflammatory processes and oxidative stress.304 To solve this issue, immunomodulatory drugs are used to modulate or modify immune responses. By stimulating or suppressing the immune system, immunoregulators normalize the production of immune cells to reduce the body’s adverse immune responses. Immunomodulators may be further divided into immunostimulants and immunosuppressants. The mechanisms for various classes of immunomodulatory drugs are different, which are listed in Table 1.305,306
Table 1.
Approved Immunomodulatory Drugs
|
Family
|
Drug
|
Mechanisms of Action
|
References
|
| Glucocorticoids |
Prednisone, Dexamethasone |
Suppress inflammatory mediators released from many immune cells such as macrophages, neutrophils, dendritic cells, mast cells, and T cells.
Repression of pro-inflammatory transcription factors such as nuclear factor-kappa B (NF-κB) and activator protein-1 (AP-1) |
307,308
|
Cytokines-based inhibitors
(interleukins and tumor necrosis factor alpha (TNF-α)-blocking agents) |
simple
-
1. Interleukins 1, 2, 6-inhibitors: anakinra, canakinumab, daclizumab, basiliximab,
-
2. TNF-alpha blocking agents:
|
Inhibition of the production of cytokines and chemokines involved in pro-inflammatory and inflammatory processes. Anti-cytokine receptors.
Block soluble TNF receptors type II and bind to TNF-alpha and TNF-beta, inhibit circulating TNF and lymphotoxin A.
A chimeric monoclonal IgG1 antibody against soluble and transmembrane TNF-α. TNF-α is a key pro-inflammatory cytokine involved in chronic inflammatory diseases.
Human recombinant monoclonal
antibody against TNF-α |
309-315
|
|
Cytostatic agents
|
simple
-
2. Antimetabolites agents:
simple
-
-
-
• Mycophenolate mofetil (MMF)
|
Binds to DNA, cross-link with the strands of DNA and RNA and inhibits protein synthesis. Inhibits proliferation of T and B lymphocytes. Effective against B cell compared to T cell.
Inhibits RNA and DNA synthesis by interfering with purine synthesis along with inhibition of B and T cells. Acts in S phase and prevents adenine and guanine synthesis. Inducing T cell apoptosis
Inhibits purine and pyrimidine synthesis, increases adenosine release; adenosine binds to cell surface receptors and suppresses many inflammatory and immune reactions. Inhibits dihydrofolate reductase, preventing the reduction of dihydrobiopterin (BH2) to tetrahydrobiopterin (BH4), leading to nitric oxide synthase uncoupling and increased sensitivity of T cells to apoptosis, thereby diminishing immune responses.
Reversible inhibitor of inosine monophosphate dehydrogenase (IMPDH) and inhibits de novo purine synthesis. Prevents the proliferation of immune cells such as B and T cells. |
316-321
|
| Antibody-specific agents |
simple
-
1) Polyclonal antibodies: Anti-thymocyte globulin (ATG)
-
2) Monoclonal antibodies:
simple
-
• Muromunab (OKT-3) (Anti- CD3 monoclonal antibody)
-
-
• Rituximab (B cell modulators)
-
• Efalizumab (Inhibitors of immune cell adhesion molecules)
-
|
Apoptosis via activation-induced cell death
Antibody-dependent cell-mediated cytotoxicity (ADCC) complement-dependent cytotoxicity (CDC)
Monomeric immune globulin G type 2a (IgG2a). Destruction of the chain of CD3 on the surface of T-cells involved in antigen recognition,
cell signaling and proliferation, thereby inhibiting subsequent antigen recognition
Humanized monoclonal antibody specific to CD52 glycoprotein.
Anti-CD 20 protein expressed on the surface of B
cells.
Humanized monoclonal antibody against CD11a, a subunit of the lymphocyte function-associated antigen 1 (LFA-1), which is present on lymphocytes and causes activation and proliferation of lymphocytes.
Anti-epidermal growth factor (EGF) receptors |
322-329
|
| Anti-lymphocyte agents (T cell modulators) |
|
Blocks the interaction between the leukocyte-function-associated antigen (LFA)-3 and CD2 and impedes the activation and proliferation of T cells. Stimulate apoptosis of activated memory T cells.
Blocks T cell costimulatory activation by binding to CD80 and CD86, blocking interaction with CD28, thereby inhibiting the activation of T lymphocytes. |
330-332
|
| Drugs that bind to the immunophilins |
simple
-
1) Calcineurin inhibitors: Cyclosporin A
-
2) mTOR inhibitors: Tacrolimus, Sirolimus
|
Reversible inhibition of immunocompetent lymphocytes in the G0- and G1-phase of the cell cycle. Forming a complex with cyclophilin to block the phosphatase activity of calcineurin which reduce the production of inflammatory cytokines by T-lymphocytes and lymphocytes signaling.
Binding to the immunophilin FKBP-12 (FK506 binding protein), thereby inhibiting both T-lymphocyte signal transduction and IL-2 transcription and cytokine release by T cells. |
333-336
|
| Antibiotics |
Dapsone (Sulfone antibiotic) |
Anti-inflammatory against PMN, inhibiting neutrophil chemotaxis by blocking myeloperoxidase. |
337,338
|
Discussion
As mentioned above, chronic inflammation and oxidants are considered the leading causes of cancer progression. We concluded that oxidative stress, as a disease-advancing reaction, can be introduced in a multifactorial context as a critical mechanism for inducing different cellular and molecular pathways that are more favorable for disease progression, inflammation, and cancer. From this study, it could be concluded that chronic inflammation and oxidative stress are described as the leading causes of cancer establishment and progression. Undoubtedly, therapeutic approaches can benefit from the neutralization of tumor-induced inflammation, and oxidative stress for the development of suitable anti-inflammatory-related drugs and the prognosis of neoplastic diseases that may improve treatment regimens against cancer progression. Further research is currently being conducted to identify the precise and constitutive mechanism that remains an exciting and challenging topic. Down-regulation of survival of ROS –induced tumor cells, attention to pro-mutagenic signals of tumor cells, and augmentation of anti-tumor immune responses that are mediated by the infiltrating and circulating effector T cells are three essential strategies that prevent cancer/inflammation-associated signals. Understanding the exact mechanisms of redox signaling, as a super-fast communication procedure, and the roles of redox enzymes is of great importance in designing suitable drugs in this field. This information can be used to manage patients with various diseases or create more effective targeted antioxidant therapy approaches for the treatment of oxidative-induced cancer and chronic inflammation.
Conclusion
This review clearly shows the role of ROS in inflammation and cancer development. Therefore, targeting redox-sensitive pathways and transcription factors could provide promising therapeutic approaches for cancer prevention and treatment in the future. Antioxidant therapy may be explored as a treatment option to reduce cancer incidence and progression.339-341 Antioxidants, as adjuvant, may positively affect cancer treatment outcomes or inhibit the adverse side effects of conventional therapies such as chemotherapy and radiotherapy.342,343 Achieving these positive results depends on several factors, including the antioxidant dose, synergism, bioavailability of consumed antioxidants, lifestyle, health status of patients, type and stage of cancer, and so on. Therefore, the therapeutic usefulness of antioxidants in the treatment of cancer still has many aspects that need to be investigated in the future.
Authors’ Contribution
Conceptualization: Somayeh Ashrafi.
Data curation: Neda Zahmatkesh.
Formal analysis: Mehdi Mahdavi.
Funding acquisition: Sara Rahimzadeh.
Investigation: Sara Rahimzadeh.
Methodology: Nastaran Hadizadeh.
Project administration: Nastaran Hadizadeh.
Resources: Sara Rahimzadeh.
Software: Nastaran Hadizadeh.
Supervision: Mohammad Abdollahi, Mohammad Hossein Yazdi.
Validation: Nastaran Hadizadeh.
Visualization: Nastaran Hadizadeh.
Writing original draft: Somayeh Ashrafi, Melika Khanzadeh Tehrani.
Writing-review & editing: Sara Rahimzadeh.
Competing Interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interests.
Ethical Approval
This study was approved by the Ethics Committee of Tehran University of Medical Sciences (IR.TUMS.DDRI.REC.1401.036).
References
- Lushchak VI. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem Biol Interact 2014; 224:164-75. doi: 10.1016/j.cbi.2014.10.016 [Crossref] [ Google Scholar]
- Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med 2001; 30(11):1191-212. doi: 10.1016/s0891-5849(01)00480-4 [Crossref] [ Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007; 39(1):44-84. doi: 10.1016/j.biocel.2006.07.001 [Crossref] [ Google Scholar]
- Dröge W. Free radicals in the physiological control of cell function. Physiol Rev 2002; 82(1):47-95. doi: 10.1152/physrev.00018.2001 [Crossref] [ Google Scholar]
- Klaunig JE, Kamendulis LM, Hocevar BA. Oxidative stress and oxidative damage in carcinogenesis. Toxicol Pathol 2010; 38(1):96-109. doi: 10.1177/0192623309356453 [Crossref] [ Google Scholar]
- Liou GY, Storz P. Reactive oxygen species in cancer. Free Radic Res 2010; 44(5):479-96. doi: 10.3109/10715761003667554 [Crossref] [ Google Scholar]
- Gupta RK, Patel AK, Kumari R, Chugh S, Shrivastav C, Mehra S. Interactions between oxidative stress, lipid profile and antioxidants in breast cancer: a case control study. Asian Pac J Cancer Prev 2012; 13(12):6295-8. doi: 10.7314/apjcp.2012.13.12.6295 [Crossref] [ Google Scholar]
- Manda G, Nechifor MT, Neagu TM. Reactive oxygen species, cancer and anti-cancer therapies. Curr Chem Biol 2009; 3(1):22-46. [ Google Scholar]
- Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 2006; 160(1):1-40. doi: 10.1016/j.cbi.2005.12.009 [Crossref] [ Google Scholar]
- Brown GC, Borutaite V. There is no evidence that mitochondria are the main source of reactive oxygen species in mammalian cells. Mitochondrion 2012; 12(1):1-4. doi: 10.1016/j.mito.2011.02.001 [Crossref] [ Google Scholar]
- Conner EM, Grisham MB. Inflammation, free radicals, and antioxidants. Nutrition 1996; 12(4):274-7. doi: 10.1016/s0899-9007(96)00000-8 [Crossref] [ Google Scholar]
- Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V. Oxidative stress: harms and benefits for human health. Oxid Med Cell Longev 2017; 2017:8416763. doi: 10.1155/2017/8416763 [Crossref] [ Google Scholar]
- Deweirdt J, Quignard JF, Crobeddu B, Baeza-Squiban A, Sciare J, Courtois A. Involvement of oxidative stress and calcium signaling in airborne particulate matter - induced damages in human pulmonary artery endothelial cells. Toxicol In Vitro 2017; 45(Pt 3):340-50. doi: 10.1016/j.tiv.2017.07.001 [Crossref] [ Google Scholar]
- Mateos R, Bravo L. Chromatographic and electrophoretic methods for the analysis of biomarkers of oxidative damage to macromolecules (DNA, lipids, and proteins). J Sep Sci 2007; 30(2):175-91. doi: 10.1002/jssc.200600314 [Crossref] [ Google Scholar]
- Simic MG, Bergtold DS, Karam LR. Generation of oxy radicals in biosystems. Mutat Res 1989; 214(1):3-12. doi: 10.1016/0027-5107(89)90192-9 [Crossref] [ Google Scholar]
- Cheeseman KH, Slater TF. An introduction to free radical biochemistry. Br Med Bull 1993; 49(3):481-93. doi: 10.1093/oxfordjournals.bmb.a072625 [Crossref] [ Google Scholar]
- Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer 2003; 3(4):276-85. doi: 10.1038/nrc1046 [Crossref] [ Google Scholar]
- Bauer G. HOCl-dependent singlet oxygen and hydroxyl radical generation modulate and induce apoptosis of malignant cells. Anticancer Res 2013; 33(9):3589-602. [ Google Scholar]
- Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked?. Free Radic Biol Med 2010; 49(11):1603-16. doi: 10.1016/j.freeradbiomed.2010.09.006 [Crossref] [ Google Scholar]
- Sosa V, Moliné T, Somoza R, Paciucci R, Kondoh H, Lleonart ME. Oxidative stress and cancer: an overview. Ageing Res Rev 2013; 12(1):376-90. doi: 10.1016/j.arr.2012.10.004 [Crossref] [ Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 2007; 87(1):315-424. doi: 10.1152/physrev.00029.2006 [Crossref] [ Google Scholar]
- Ryan KA, Smith MF Jr, Sanders MK, Ernst PB. Reactive oxygen and nitrogen species differentially regulate toll-like receptor 4-mediated activation of NF-κB and interleukin-8 expression. Infect Immun 2004; 72(4):2123-30. doi: 10.1128/iai.72.4.2123-2130.2004 [Crossref] [ Google Scholar]
- Okin D, Medzhitov R. Evolution of inflammatory diseases. Curr Biol 2012; 22(17):R733-40. doi: 10.1016/j.cub.2012.07.029 [Crossref] [ Google Scholar]
- Muller WA. Getting leukocytes to the site of inflammation. Vet Pathol 2013; 50(1):7-22. doi: 10.1177/0300985812469883 [Crossref] [ Google Scholar]
- Dmitrieva OS, Shilovskiy IP, Khaitov MR, Grivennikov SI. Interleukins 1 and 6 as main mediators of inflammation and cancer. Biochemistry (Mosc) 2016; 81(2):80-90. doi: 10.1134/s0006297916020024 [Crossref] [ Google Scholar]
- Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 2013; 13(3):159-75. doi: 10.1038/nri3399 [Crossref] [ Google Scholar]
- Bartsch H, Nair J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: role of lipid peroxidation, DNA damage, and repair. Langenbecks Arch Surg 2006; 391(5):499-510. doi: 10.1007/s00423-006-0073-1 [Crossref] [ Google Scholar]
- Azad N, Rojanasakul Y, Vallyathan V. Inflammation and lung cancer: roles of reactive oxygen/nitrogen species. J Toxicol Environ Health B Crit Rev 2008; 11(1):1-15. doi: 10.1080/10937400701436460 [Crossref] [ Google Scholar]
- Aranda-Rivera AK, Cruz-Gregorio A, Arancibia-Hernández YL, Hernández-Cruz EY, Pedraza-Chaverri J. RONS and oxidative stress: an overview of basic concepts. Oxygen 2022; 2(4):437-78. doi: 10.3390/oxygen2040030 [Crossref] [ Google Scholar]
- Steinbrenner H, Sies H. Protection against reactive oxygen species by selenoproteins. Biochim Biophys Acta 2009; 1790(11):1478-85. doi: 10.1016/j.bbagen.2009.02.014 [Crossref] [ Google Scholar]
- Chen AF, Chen DD, Daiber A, Faraci FM, Li H, Rembold CM. Free radical biology of the cardiovascular system. Clin Sci (Lond) 2012; 123(2):73-91. doi: 10.1042/cs20110562 [Crossref] [ Google Scholar]
- Bouayed J, Bohn T. Exogenous antioxidants--double-edged swords in cellular redox state: health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid Med Cell Longev 2010; 3(4):228-37. doi: 10.4161/oxim.3.4.12858 [Crossref] [ Google Scholar]
- Snezhkina AV, Kudryavtseva AV, Kardymon OL, Savvateeva MV, Melnikova NV, Krasnov GS. ROS generation and antioxidant defense systems in normal and malignant cells. Oxid Med Cell Longev 2019; 2019:6175804. doi: 10.1155/2019/6175804 [Crossref] [ Google Scholar]
- Kurutas EB. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr J 2016; 15(1):71. doi: 10.1186/s12937-016-0186-5 [Crossref] [ Google Scholar]
- Carocho M, Ferreira IC. A review on antioxidants, prooxidants and related controversy: natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem Toxicol 2013; 51:15-25. doi: 10.1016/j.fct.2012.09.021 [Crossref] [ Google Scholar]
- Owen JD, Strieter R, Burdick M, Haghnegahdar H, Nanney L, Shattuck-Brandt R. Enhanced tumor-forming capacity for immortalized melanocytes expressing melanoma growth stimulatory activity/growth-regulated cytokine beta and gamma proteins. Int J Cancer 1997; 73(1):94-103. doi: 10.1002/(sici)1097-0215(19970926)73:1<94::aidijc15>3.0.co;2-5 [Crossref] [ Google Scholar]
- Shao D, Oka S, Brady CD, Haendeler J, Eaton P, Sadoshima J. Redox modification of cell signaling in the cardiovascular system. J Mol Cell Cardiol 2012; 52(3):550-8. doi: 10.1016/j.yjmcc.2011.09.009 [Crossref] [ Google Scholar]
- Nathan C, Ding A. Nonresolving inflammation. Cell 2010; 140(6):871-82. doi: 10.1016/j.cell.2010.02.029 [Crossref] [ Google Scholar]
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell 2010; 140(6):805-20. doi: 10.1016/j.cell.2010.01.022 [Crossref] [ Google Scholar]
- Tse KH, Chow KB, Leung WK, Wong YH, Wise H. Lipopolysaccharide differentially modulates expression of cytokines and cyclooxygenases in dorsal root ganglion cells via toll-like receptor-4 dependent pathways. Neuroscience 2014; 267:241-51. doi: 10.1016/j.neuroscience.2014.02.041 [Crossref] [ Google Scholar]
- Savill JS, Wyllie AH, Henson JE, Walport MJ, Henson PM, Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest 1989; 83(3):865-75. doi: 10.1172/jci113970 [Crossref] [ Google Scholar]
- Kumar R, Clermont G, Vodovotz Y, Chow CC. The dynamics of acute inflammation. J Theor Biol 2004; 230(2):145-55. doi: 10.1016/j.jtbi.2004.04.044 [Crossref] [ Google Scholar]
- Gatti G, Rivero V, Motrich RD, Maccioni M. Prostate epithelial cells can act as early sensors of infection by up-regulating TLR4 expression and proinflammatory mediators upon LPS stimulation. J Leukoc Biol 2006; 79(5):989-98. doi: 10.1189/jlb.1005597 [Crossref] [ Google Scholar]
- Medzhitov R. Innate immunity: quo vadis?. Nat Immunol 2010; 11(7):551-3. doi: 10.1038/ni0710-551 [Crossref] [ Google Scholar]
- Barton GM. A calculated response: control of inflammation by the innate immune system. J Clin Invest 2008; 118(2):413-20. doi: 10.1172/jci34431 [Crossref] [ Google Scholar]
- Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol 2013; 31:563-604. doi: 10.1146/annurev-immunol-020711-074950 [Crossref] [ Google Scholar]
- Shalapour S, Karin M. Pas de deux: control of anti-tumor immunity by cancer-associated inflammation. Immunity 2019; 51(1):15-26. doi: 10.1016/j.immuni.2019.06.021 [Crossref] [ Google Scholar]
- Lugrin J, Rosenblatt-Velin N, Parapanov R, Liaudet L. The role of oxidative stress during inflammatory processes. Biol Chem 2014; 395(2):203-30. doi: 10.1515/hsz-2013-0241 [Crossref] [ Google Scholar]
- Boyle M, Chun C, Strojny C, Narayanan R, Bartholomew A, Sundivakkam P. Chronic inflammation and angiogenic signaling axis impairs differentiation of dental-pulp stem cells. PLoS One 2014; 9(11):e113419. doi: 10.1371/journal.pone.0113419 [Crossref] [ Google Scholar]
- Feehan KT, Gilroy DW. Is resolution the end of inflammation?. Trends Mol Med 2019; 25(3):198-214. doi: 10.1016/j.molmed.2019.01.006 [Crossref] [ Google Scholar]
- Serhan CN. Controlling the resolution of acute inflammation: a new genus of dual anti-inflammatory and proresolving mediators. J Periodontol 2008; 79(8 Suppl):1520-6. doi: 10.1902/jop.2008.080231 [Crossref] [ Google Scholar]
- Ploeger HE, Takken T, de Greef MH, Timmons BW. The effects of acute and chronic exercise on inflammatory markers in children and adults with a chronic inflammatory disease: a systematic review. Exerc Immunol Rev 2009; 15:6-41. [ Google Scholar]
- Bozkurt B, Mann DL, Deswal A. Biomarkers of inflammation in heart failure. Heart Fail Rev 2010; 15(4):331-41. doi: 10.1007/s10741-009-9140-3 [Crossref] [ Google Scholar]
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell 2010; 140(6):918-34. doi: 10.1016/j.cell.2010.02.016 [Crossref] [ Google Scholar]
- Kawanishi S, Ohnishi S, Ma N, Hiraku Y, Murata M. Crosstalk between DNA damage and inflammation in the multiple steps of carcinogenesis. Int J Mol Sci 2017; 18(8):1808. doi: 10.3390/ijms18081808 [Crossref] [ Google Scholar]
- Eiró N, Vizoso FJ. Inflammation and cancer. World J Gastrointest Surg 2012; 4(3):62-72. doi: 10.4240/wjgs.v4.i3.62 [Crossref] [ Google Scholar]
- Cook JA, Gius D, Wink DA, Krishna MC, Russo A, Mitchell JB. Oxidative stress, redox, and the tumor microenvironment. Semin Radiat Oncol 2004; 14(3):259-66. doi: 10.1016/j.semradonc.2004.04.001 [Crossref] [ Google Scholar]
- Zhao H, Wu L, Yan G, Chen Y, Zhou M, Wu Y. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther 2021; 6(1):263. doi: 10.1038/s41392-021-00658-5 [Crossref] [ Google Scholar]
- Khansari N, Shakiba Y, Mahmoudi M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat Inflamm Allergy Drug Discov 2009; 3(1):73-80. doi: 10.2174/187221309787158371 [Crossref] [ Google Scholar]
- Liaudet L, Vassalli G, Pacher P. Role of peroxynitrite in the redox regulation of cell signal transduction pathways. Front Biosci (Landmark Ed) 2009; 14(12):4809-14. doi: 10.2741/3569 [Crossref] [ Google Scholar]
- Zhang J, Nakayama J, Ohyama C, Suzuki M, Suzuki A, Fukuda M. Sialyl Lewis X-dependent lung colonization of B16 melanoma cells through a selectin-like endothelial receptor distinct from E- or P-selectin. Cancer Res 2002; 62(15):4194-8. [ Google Scholar]
- Meira LB, Bugni JM, Green SL, Lee CW, Pang B, Borenshtein D. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J Clin Invest 2008; 118(7):2516-25. doi: 10.1172/jci35073 [Crossref] [ Google Scholar]
- Qian F, Hanahan D, Weissman IL. L-selectin can facilitate metastasis to lymph nodes in a transgenic mouse model of carcinogenesis. Proc Natl Acad Sci U S A 2001; 98(7):3976-81. doi: 10.1073/pnas.061633698 [Crossref] [ Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature 2000; 408(6809):239-47. doi: 10.1038/35041687 [Crossref] [ Google Scholar]
- Chan JK, Roth J, Oppenheim JJ, Tracey KJ, Vogl T, Feldmann M. Alarmins: awaiting a clinical response. J Clin Invest 2012; 122(8):2711-9. doi: 10.1172/jci62423 [Crossref] [ Google Scholar]
- Saïd-Sadier N, Ojcius DM. Alarmins, inflammasomes and immunity. Biomed J 2012; 35(6):437-49. doi: 10.4103/2319-4170.104408 [Crossref] [ Google Scholar]
- Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van Loo G. Identification of oxidative stress and toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 2008; 133(2):235-49. doi: 10.1016/j.cell.2008.02.043 [Crossref] [ Google Scholar]
- Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol 2008; 8(4):279-89. doi: 10.1038/nri2215 [Crossref] [ Google Scholar]
- Miller YI, Choi SH, Wiesner P, Fang L, Harkewicz R, Hartvigsen K. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res 2011; 108(2):235-48. doi: 10.1161/circresaha.110.223875 [Crossref] [ Google Scholar]
- Chou MY, Hartvigsen K, Hansen LF, Fogelstrand L, Shaw PX, Boullier A. Oxidation-specific epitopes are important targets of innate immunity. J Intern Med 2008; 263(5):479-88. doi: 10.1111/j.1365-2796.2008.01968.x [Crossref] [ Google Scholar]
- Kampfrath T, Maiseyeu A, Ying Z, Shah Z, Deiuliis JA, Xu X. Chronic fine particulate matter exposure induces systemic vascular dysfunction via NADPH oxidase and TLR4 pathways. Circ Res 2011; 108(6):716-26. doi: 10.1161/circresaha.110.237560 [Crossref] [ Google Scholar]
- Choi SH, Harkewicz R, Lee JH, Boullier A, Almazan F, Li AC. Lipoprotein accumulation in macrophages via toll-like receptor-4-dependent fluid phase uptake. Circ Res 2009; 104(12):1355-63. doi: 10.1161/circresaha.108.192880 [Crossref] [ Google Scholar]
- Lahoute C, Herbin O, Mallat Z, Tedgui A. Adaptive immunity in atherosclerosis: mechanisms and future therapeutic targets. Nat Rev Cardiol 2011; 8(6):348-58. doi: 10.1038/nrcardio.2011.62 [Crossref] [ Google Scholar]
- Greig FH, Kennedy S, Spickett CM. Physiological effects of oxidized phospholipids and their cellular signaling mechanisms in inflammation. Free Radic Biol Med 2012; 52(2):266-80. doi: 10.1016/j.freeradbiomed.2011.10.481 [Crossref] [ Google Scholar]
- Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol 2001; 13(1):114-9. doi: 10.1016/s0952-7915(00)00191-6 [Crossref] [ Google Scholar]
- Lee S, Birukov KG, Romanoski CE, Springstead JR, Lusis AJ, Berliner JA. Role of phospholipid oxidation products in atherosclerosis. Circ Res 2012; 111(6):778-99. doi: 10.1161/circresaha.111.256859 [Crossref] [ Google Scholar]
- Leibundgut G, Witztum JL, Tsimikas S. Oxidation-specific epitopes and immunological responses: translational biotheranostic implications for atherosclerosis. Curr Opin Pharmacol 2013; 13(2):168-79. doi: 10.1016/j.coph.2013.02.005 [Crossref] [ Google Scholar]
- Singh N, Baby D, Rajguru JP, Patil PB, Thakkannavar SS, Pujari VB. Inflammation and cancer. Ann Afr Med 2019; 18(3):121-6. doi: 10.4103/aam.aam_56_18 [Crossref] [ Google Scholar]
- Yang J, Yang X, Zou H, Li M. Oxidative stress and Treg and Th17 dysfunction in systemic lupus erythematosus. Oxid Med Cell Longev 2016; 2016:2526174. doi: 10.1155/2016/2526174 [Crossref] [ Google Scholar]
- Kauppinen A, Paterno JJ, Blasiak J, Salminen A, Kaarniranta K. Inflammation and its role in age-related macular degeneration. Cell Mol Life Sci 2016; 73(9):1765-86. doi: 10.1007/s00018-016-2147-8 [Crossref] [ Google Scholar]
- Korniluk A, Koper O, Kemona H, Dymicka-Piekarska V. From inflammation to cancer. Ir J Med Sci 2017; 186(1):57-62. doi: 10.1007/s11845-016-1464-0 [Crossref] [ Google Scholar]
- Hayes JD, Dinkova-Kostova AT, Tew KD. Oxidative stress in cancer. Cancer Cell 2020; 38(2):167-97. doi: 10.1016/j.ccell.2020.06.001 [Crossref] [ Google Scholar]
- Naik E, Dixit VM. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J Exp Med 2011; 208(3):417-20. doi: 10.1084/jem.20110367 [Crossref] [ Google Scholar]
- Kamp DW, Shacter E, Weitzman SA. Chronic inflammation and cancer: the role of the mitochondria. Oncology (Williston Park) 2011; 25(5):400-10. [ Google Scholar]
- Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer 2007; 121(11):2373-80. doi: 10.1002/ijc.23173 [Crossref] [ Google Scholar]
- Weidemüller P, Kholmatov M, Petsalaki E, Zaugg JB. Transcription factors: bridge between cell signaling and gene regulation. Proteomics 2021; 21(23-24):e2000034. doi: 10.1002/pmic.202000034 [Crossref] [ Google Scholar]
- Federico A, Morgillo F, Tuccillo C, Ciardiello F, Loguercio C. Chronic inflammation and oxidative stress in human carcinogenesis. Int J Cancer 2007; 121(11):2381-6. doi: 10.1002/ijc.23192 [Crossref] [ Google Scholar]
- Sklyarov AY, Panasyuk NB, Fomenko IS. Role of nitric oxide-synthase and cyclooxygenase/lipooxygenase systems in development of experimental ulcerative colitis. J Physiol Pharmacol 2011; 62(1):65-73. [ Google Scholar]
- Iorio MV, Croce CM. MicroRNA dysregulation in cancer: diagnostics, monitoring and therapeutics A comprehensive review. EMBO Mol Med 2012; 4(3):143-59. doi: 10.1002/emmm.201100209 [Crossref] [ Google Scholar]
- Crawford S. Anti-inflammatory/antioxidant use in long-term maintenance cancer therapy: a new therapeutic approach to disease progression and recurrence. Ther Adv Med Oncol 2014; 6(2):52-68. doi: 10.1177/1758834014521111 [Crossref] [ Google Scholar]
- Kim MK, Song YS. Stress response, inflammaging, and cancer. In: Rahman I, Bagchi D, eds. Inflammation, Advancing Age and Nutrition. San Diego: Academic Press; 2014. p. 49-53. 10.1016/b978-0-12-397803-5.00005-8.
- De Simone V, Franzè E, Ronchetti G, Colantoni A, Fantini MC, Di Fusco D. Th17-type cytokines, IL-6 and TNF-α synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene 2015; 34(27):3493-503. doi: 10.1038/onc.2014.286 [Crossref] [ Google Scholar]
- Civenni G, Shinde D, Zoma M, Albino D, Costales P, Moris F. The multi-kinase inhibitor EC-70124 delivers a double-hit to prostate cancer stem cells interfering with both STAT3 and NF-kB signaling. Eur Urol Suppl 2017; 3(16):e1294. doi: 10.1016/s1569-9056(17)30803-5 [Crossref] [ Google Scholar]
- Daniluk J, Liu Y, Deng D, Chu J, Huang H, Gaiser S. An NF-κB pathway-mediated positive feedback loop amplifies Ras activity to pathological levels in mice. J Clin Invest 2012; 122(4):1519-28. doi: 10.1172/jci59743 [Crossref] [ Google Scholar]
- Park MT, Kim MJ, Suh Y, Kim RK, Kim H, Lim EJ. Novel signaling axis for ROS generation during K-Ras-induced cellular transformation. Cell Death Differ 2014; 21(8):1185-97. doi: 10.1038/cdd.2014.34 [Crossref] [ Google Scholar]
- Maciag A, Sithanandam G, Anderson LM. Mutant K-rasV12 increases COX-2, peroxides and DNA damage in lung cells. Carcinogenesis 2004; 25(11):2231-7. doi: 10.1093/carcin/bgh245 [Crossref] [ Google Scholar]
- Bos JL. ras oncogenes in human cancer: a review. Cancer Res 1989; 49(17):4682-9. [ Google Scholar]
- Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell 2004; 6(5):447-58. doi: 10.1016/j.ccr.2004.09.028 [Crossref] [ Google Scholar]
- Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res 2014; 2014:149185. doi: 10.1155/2014/149185 [Crossref] [ Google Scholar]
- Di Minin G, Bellazzo A, Dal Ferro M, Chiaruttini G, Nuzzo S, Bicciato S. Mutant p53 reprograms TNF signaling in cancer cells through interaction with the tumor suppressor DAB2IP. Mol Cell 2014; 56(5):617-29. doi: 10.1016/j.molcel.2014.10.013 [Crossref] [ Google Scholar]
- Candido J, Hagemann T. Cancer-related inflammation. J Clin Immunol 2013; 33 Suppl 1:S79-84. doi: 10.1007/s10875-012-9847-0 [Crossref] [ Google Scholar]
- Simons A, Stanam A, Koch A, Espinosa-Cotton M, Gibson-Corley K. Targeting IL-1 signaling to improve tumor response to erlotinib in HNSCC. Clin Cancer Res 2017; 23(23 Suppl):48. doi: 10.1158/1557-3265.aacrahns17-48 [Crossref] [ Google Scholar]
- Grivennikov SI, Karin M. Inflammation and oncogenesis: a vicious connection. Curr Opin Genet Dev 2010; 20(1):65-71. doi: 10.1016/j.gde.2009.11.004 [Crossref] [ Google Scholar]
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow?. Lancet 2001; 357(9255):539-45. doi: 10.1016/s0140-6736(00)04046-0 [Crossref] [ Google Scholar]
- Liu X, Yin L, Shen S, Hou Y. Inflammation and cancer: paradoxical roles in tumorigenesis and implications in immunotherapies. Genes Dis 2023; 10(1):151-64. doi: 10.1016/j.gendis.2021.09.006 [Crossref] [ Google Scholar]
- Salem ML, Attia ZI, Galal SM. Acute inflammation induces immunomodulatory effects on myeloid cells associated with anti-tumor responses in a tumor mouse model. J Adv Res 2016; 7(2):243-53. doi: 10.1016/j.jare.2015.06.001 [Crossref] [ Google Scholar]
- Surace L, Scheifinger NA, Gupta A, van den Broek M. Radiotherapy supports tumor-specific immunity by acute inflammation. Oncoimmunology 2016; 5(1):e1060391. doi: 10.1080/2162402x.2015.1060391 [Crossref] [ Google Scholar]
- Urban-Wojciuk Z, Khan MM, Oyler BL, Fåhraeus R, Marek-Trzonkowska N, Nita-Lazar A. The role of TLRs in anti-cancer immunity and tumor rejection. Front Immunol 2019; 10:2388. doi: 10.3389/fimmu.2019.02388 [Crossref] [ Google Scholar]
- Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology (Williston Park) 2002; 16(2):217-26. [ Google Scholar]
- Multhoff G, Molls M, Radons J. Chronic inflammation in cancer development. Front Immunol 2011; 2:98. doi: 10.3389/fimmu.2011.00098 [Crossref] [ Google Scholar]
- Richards CH, Roxburgh CS, Powell AG, Foulis AK, Horgan PG, McMillan DC. The clinical utility of the local inflammatory response in colorectal cancer. Eur J Cancer 2014; 50(2):309-19. doi: 10.1016/j.ejca.2013.09.008 [Crossref] [ Google Scholar]
- Zhao Y, Ge X, He J, Cheng Y, Wang Z, Wang J. The prognostic value of tumor-infiltrating lymphocytes in colorectal cancer differs by anatomical subsite: a systematic review and meta-analysis. World J Surg Oncol 2019; 17(1):85. doi: 10.1186/s12957-019-1621-9 [Crossref] [ Google Scholar]
- Lam M, Tie J, Lee B, Desai J, Gibbs P, Tran B. Systemic inflammation–impact on tumor biology and outcomes in colorectal cancer. J Clin Cell Immunol 2015; 6:377. doi: 10.4172/2155-9899.1000377 [Crossref] [ Google Scholar]
- Chen JH, Zhai ET, Yuan YJ, Wu KM, Xu JB, Peng JJ. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol 2017; 23(34):6261-72. doi: 10.3748/wjg.v23.i34.6261 [Crossref] [ Google Scholar]
- Huang L, Liu S, Lei Y, Wang K, Xu M, Chen Y. Systemic immune-inflammation index, thymidine phosphorylase and survival of localized gastric cancer patients after curative resection. Oncotarget 2016; 7(28):44185-93. doi: 10.18632/oncotarget.9923 [Crossref] [ Google Scholar]
- Kazemi MH, Sadri M, Najafi A, Rahimi A, Baghernejadan Z, Khorramdelazad H. Tumor-infiltrating lymphocytes for treatment of solid tumors: it takes two to tango?. Front Immunol 2022; 13:1018962. doi: 10.3389/fimmu.2022.1018962 [Crossref] [ Google Scholar]
- Rossi S, Basso M, Strippoli A, Schinzari G, D’Argento E, Larocca M. Are markers of systemic inflammation good prognostic indicators in colorectal cancer?. Clin Colorectal Cancer 2017; 16(4):264-74. doi: 10.1016/j.clcc.2017.03.015 [Crossref] [ Google Scholar]
- Vacca P, Munari E, Tumino N, Moretta F, Pietra G, Vitale M. Human natural killer cells and other innate lymphoid cells in cancer: friends or foes?. Immunol Lett 2018; 201:14-9. doi: 10.1016/j.imlet.2018.11.004 [Crossref] [ Google Scholar]
- Chrisikos TT, Zhou Y, Slone N, Babcock R, Watowich SS, Li HS. Molecular regulation of dendritic cell development and function in homeostasis, inflammation, and cancer. Mol Immunol 2019; 110:24-39. doi: 10.1016/j.molimm.2018.01.014 [Crossref] [ Google Scholar]
- Panigrahy D, Gartung A, Yang J, Yang H, Gilligan MM, Sulciner ML. Preoperative stimulation of resolution and inflammation blockade eradicates micrometastases. J Clin Invest 2019; 129(7):2964-79. doi: 10.1172/jci127282 [Crossref] [ Google Scholar]
- Llitjos JF, Auffray C, Alby-Laurent F, Rousseau C, Merdji H, Bonilla N. Sepsis-induced expansion of granulocytic myeloid-derived suppressor cells promotes tumour growth through toll-like receptor 4. J Pathol 2016; 239(4):473-83. doi: 10.1002/path.4744 [Crossref] [ Google Scholar]
- Cavassani KA, Carson WF 4th, Moreira AP, Wen H, Schaller MA, Ishii M. The post sepsis-induced expansion and enhanced function of regulatory T cells create an environment to potentiate tumor growth. Blood 2010; 115(22):4403-11. doi: 10.1182/blood-2009-09-241083 [Crossref] [ Google Scholar]
- Malekghasemi S, Majidi J, Baghbanzadeh A, Abdolalizadeh J, Baradaran B, Aghebati-Maleki L. Tumor-associated macrophages: protumoral macrophages in inflammatory tumor microenvironment. Adv Pharm Bull 2020; 10(4):556-65. doi: 10.34172/apb.2020.066 [Crossref] [ Google Scholar]
- Balkwill F. TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev 2006; 25(3):409-16. doi: 10.1007/s10555-006-9005-3 [Crossref] [ Google Scholar]
- Sakamoto K, Maeda S, Hikiba Y, Nakagawa H, Hayakawa Y, Shibata W. Constitutive NF-κB activation in colorectal carcinoma plays a key role in angiogenesis, promoting tumor growth. Clin Cancer Res 2009; 15(7):2248-58. doi: 10.1158/1078-0432.Ccr-08-1383 [Crossref] [ Google Scholar]
- Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer 2009; 9(5):361-71. doi: 10.1038/nrc2628 [Crossref] [ Google Scholar]
- Montfort A, Colacios C, Levade T, Andrieu-Abadie N, Meyer N, Ségui B. The TNF paradox in cancer progression and immunotherapy. Front Immunol 2019; 10:1818. doi: 10.3389/fimmu.2019.01818 [Crossref] [ Google Scholar]
- Alotaibi AG, Li JV, Gooderham NJ. Tumour necrosis factor-α (TNF-α) enhances dietary carcinogen-induced DNA damage in colorectal cancer epithelial cells through activation of JNK signaling pathway. Toxicology 2021; 457:152806. doi: 10.1016/j.tox.2021.152806 [Crossref] [ Google Scholar]
- Zhang S, Lin ZN, Yang CF, Shi X, Ong CN, Shen HM. Suppressed NF-κB and sustained JNK activation contribute to the sensitization effect of parthenolide to TNF-alpha-induced apoptosis in human cancer cells. Carcinogenesis 2004; 25(11):2191-9. doi: 10.1093/carcin/bgh234 [Crossref] [ Google Scholar]
- Ruiz A, Palacios Y, Garcia I, Chavez-Galan L. Transmembrane TNF and its receptors TNFR1 and TNFR2 in mycobacterial infections. Int J Mol Sci 2021; 22(11):5461. doi: 10.3390/ijms22115461 [Crossref] [ Google Scholar]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010; 140(6):883-99. doi: 10.1016/j.cell.2010.01.025 [Crossref] [ Google Scholar]
- Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer 2013; 13(11):759-71. doi: 10.1038/nrc3611 [Crossref] [ Google Scholar]
- Punt S, Dronkers EA, Welters MJ, Goedemans R, Koljenović S, Bloemena E. A beneficial tumor microenvironment in oropharyngeal squamous cell carcinoma is characterized by a high T cell and low IL-17 + cell frequency. Cancer Immunol Immunother 2016; 65(4):393-403. doi: 10.1007/s00262-016-1805-x [Crossref] [ Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144(5):646-74. doi: 10.1016/j.cell.2011.02.013 [Crossref] [ Google Scholar]
- Williams CS, Mann M, DuBois RN. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene 1999; 18(55):7908-16. doi: 10.1038/sj.onc.1203286 [Crossref] [ Google Scholar]
- Balkwill F. Tumor necrosis factor or tumor promoting factor?. Cytokine Growth Factor Rev 2002; 13(2):135-41. doi: 10.1016/s1359-6101(01)00020-x [Crossref] [ Google Scholar]
- Ritter B, Greten FR. Modulating inflammation for cancer therapy. J Exp Med 2019; 216(6):1234-43. doi: 10.1084/jem.20181739 [Crossref] [ Google Scholar]
- Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol 2014; 15(11):e493-503. doi: 10.1016/s1470-2045(14)70263-3 [Crossref] [ Google Scholar]
- Kimm MA, Klenk C, Alunni-Fabbroni M, Kästle S, Stechele M, Ricke J. Tumor-associated macrophages-implications for molecular oncology and imaging. Biomedicines 2021; 9(4):374. doi: 10.3390/biomedicines9040374 [Crossref] [ Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature 2002; 420(6917):860-7. doi: 10.1038/nature01322 [Crossref] [ Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 2008; 454(7203):436-44. doi: 10.1038/nature07205 [Crossref] [ Google Scholar]
- Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 2009; 30(7):1073-81. doi: 10.1093/carcin/bgp127 [Crossref] [ Google Scholar]
- Allavena P, Garlanda C, Borrello MG, Sica A, Mantovani A. Pathways connecting inflammation and cancer. Curr Opin Genet Dev 2008; 18(1):3-10. doi: 10.1016/j.gde.2008.01.003 [Crossref] [ Google Scholar]
- García-Sánchez A, Miranda-Díaz AG, Cardona-Muñoz EG. The role of oxidative stress in physiopathology and pharmacological treatment with pro- and antioxidant properties in chronic diseases. Oxid Med Cell Longev 2020; 2020:2082145. doi: 10.1155/2020/2082145 [Crossref] [ Google Scholar]
- Spill F, Reynolds DS, Kamm RD, Zaman MH. Impact of the physical microenvironment on tumor progression and metastasis. Curr Opin Biotechnol 2016; 40:41-8. doi: 10.1016/j.copbio.2016.02.007 [Crossref] [ Google Scholar]
- Del Prete A, Schioppa T, Tiberio L, Stabile H, Sozzani S. Leukocyte trafficking in tumor microenvironment. Curr Opin Pharmacol 2017; 35:40-7. doi: 10.1016/j.coph.2017.05.004 [Crossref] [ Google Scholar]
- Tie Y, Tang F, Wei YQ, Wei XW. Immunosuppressive cells in cancer: mechanisms and potential therapeutic targets. J Hematol Oncol 2022; 15(1):61. doi: 10.1186/s13045-022-01282-8 [Crossref] [ Google Scholar]
- Yuan Z, Liang Z, Yi J, Chen X, Li R, Wu J. Koumine promotes ROS production to suppress hepatocellular carcinoma cell proliferation via NF-κB and ERK/p38 MAPK signaling. Biomolecules 2019; 9(10):559. doi: 10.3390/biom9100559 [Crossref] [ Google Scholar]
- Shimizu K, Iyoda T, Okada M, Yamasaki S, Fujii SI. Immune suppression and reversal of the suppressive tumor microenvironment. Int Immunol 2018; 30(10):445-54. doi: 10.1093/intimm/dxy042 [Crossref] [ Google Scholar]
- Fiaschi T, Chiarugi P. Oxidative stress, tumor microenvironment, and metabolic reprogramming: a diabolic liaison. Int J Cell Biol 2012; 2012:762825. doi: 10.1155/2012/762825 [Crossref] [ Google Scholar]
- Storz P. Reactive oxygen species in tumor progression. Front Biosci 2005; 10:1881-96. doi: 10.2741/1667 [Crossref] [ Google Scholar]
- Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res 1991; 51(3):794-8. [ Google Scholar]
- Sabharwal SS, Schumacker PT. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles’ heel?. Nat Rev Cancer 2014; 14(11):709-21. doi: 10.1038/nrc3803 [Crossref] [ Google Scholar]
- Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol 2014; 24(10):R453-62. doi: 10.1016/j.cub.2014.03.034 [Crossref] [ Google Scholar]
- Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J. Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem 2004; 266(1-2):37-56. doi: 10.1023/b:mcbi.0000049134.69131.89 [Crossref] [ Google Scholar]
- Huang YJ, Nan GX. Oxidative stress-induced angiogenesis. J Clin Neurosci 2019; 63:13-6. doi: 10.1016/j.jocn.2019.02.019 [Crossref] [ Google Scholar]
- Sotgia F, Martinez-Outschoorn UE, Lisanti MP. Mitochondrial oxidative stress drives tumor progression and metastasis: should we use antioxidants as a key component of cancer treatment and prevention?. BMC Med 2011; 9:62. doi: 10.1186/1741-7015-9-62 [Crossref] [ Google Scholar]
- Auyeung KK, Ko JK. Angiogenesis and oxidative stress in metastatic tumor progression: pathogenesis and novel therapeutic approach of colon cancer. Curr Pharm Des 2017; 23(27):3952-61. doi: 10.2174/1381612823666170228124105 [Crossref] [ Google Scholar]
- nokuma T, Haraguchi M, Fujita F, Tajima Y, Kanematsu T. Oxidative stress and tumor progression in colorectal cancer. Hepatogastroenterology 2009; 56(90):343-7. [ Google Scholar]
- McLoughlin MR, Orlicky DJ, Prigge JR, Krishna P, Talago EA, Cavigli IR. TrxR1, Gsr, and oxidative stress determine hepatocellular carcinoma malignancy. Proc Natl Acad Sci U S A 2019; 116(23):11408-17. doi: 10.1073/pnas.1903244116 [Crossref] [ Google Scholar]
- Huang R, Chen H, Liang J, Li Y, Yang J, Luo C. Dual role of reactive oxygen species and their application in cancer therapy. J Cancer 2021; 12(18):5543-61. doi: 10.7150/jca.54699 [Crossref] [ Google Scholar]
- Hecht F, Pessoa CF, Gentile LB, Rosenthal D, Carvalho DP, Fortunato RS. The role of oxidative stress on breast cancer development and therapy. Tumour Biol 2016; 37(4):4281-91. doi: 10.1007/s13277-016-4873-9 [Crossref] [ Google Scholar]
- Yousefi F, Asadikaram G, Karamouzian S, Abolhassani M, Pourghadamyari H, Moazed V. Organochlorine and organophosphorus pesticides may induce brain cancer through oxidative stress. Toxicol Ind Health 2022; 38(11):717-32. doi: 10.1177/07482337221125954 [Crossref] [ Google Scholar]
- Filaire E, Dupuis C, Galvaing G, Aubreton S, Laurent H, Richard R. Lung cancer: what are the links with oxidative stress, physical activity and nutrition. Lung Cancer 2013; 82(3):383-9. doi: 10.1016/j.lungcan.2013.09.009 [Crossref] [ Google Scholar]
- Moloney JN, Cotter TG. ROS signalling in the biology of cancer. Semin Cell Dev Biol 2018; 80:50-64. doi: 10.1016/j.semcdb.2017.05.023 [Crossref] [ Google Scholar]
- Canli Ö, Nicolas AM, Gupta J, Finkelmeier F, Goncharova O, Pesic M, et al. Myeloid cell-derived reactive oxygen species induce epithelial mutagenesis. Cancer Cell 2017;32(6):869-83.e5. 10.1016/j.ccell.2017.11.004.
- Jackson JH. Potential molecular mechanisms of oxidant-induced carcinogenesis. Environ Health Perspect 1994; 102(Suppl 10):155-7. doi: 10.1289/ehp.94102s10155 [Crossref] [ Google Scholar]
- Zhong LM, Liu ZG, Zhou X, Song SH, Weng GY, Wen Y. Expansion of PMN-myeloid derived suppressor cells and their clinical relevance in patients with oral squamous cell carcinoma. Oral Oncol 2019; 95:157-63. doi: 10.1016/j.oraloncology.2019.06.004 [Crossref] [ Google Scholar]
- Harmon C, Robinson MW, Hand F, Almuaili D, Mentor K, Houlihan DD. Lactate-mediated acidification of tumor microenvironment induces apoptosis of liver-resident NK cells in colorectal liver metastasis. Cancer Immunol Res 2019; 7(2):335-46. doi: 10.1158/2326-6066.Cir-18-0481 [Crossref] [ Google Scholar]
- Zhang J, Li H, Wu Q, Chen Y, Deng Y, Yang Z. Tumoral NOX4 recruits M2 tumor-associated macrophages via ROS/PI3K signaling-dependent various cytokine production to promote NSCLC growth. Redox Biol 2019; 22:101116. doi: 10.1016/j.redox.2019.101116 [Crossref] [ Google Scholar]
- Zhang Y, Choksi S, Chen K, Pobezinskaya Y, Linnoila I, Liu ZG. ROS play a critical role in the differentiation of alternatively activated macrophages and the occurrence of tumor-associated macrophages. Cell Res 2013; 23(7):898-914. doi: 10.1038/cr.2013.75 [Crossref] [ Google Scholar]
- Verfaillie T, Rubio N, Garg AD, Bultynck G, Rizzuto R, Decuypere JP. PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS-based ER stress. Cell Death Differ 2012; 19(11):1880-91. doi: 10.1038/cdd.2012.74 [Crossref] [ Google Scholar]
- Wang J, Deng H, Zhang J, Wu D, Li J, Ma J. α-Hederin induces the apoptosis of gastric cancer cells accompanied by glutathione decrement and reactive oxygen species generation via activating mitochondrial dependent pathway. Phytother Res 2020; 34(3):601-11. doi: 10.1002/ptr.6548 [Crossref] [ Google Scholar]
- Punganuru SR, Madala HR, Arutla V, Srivenugopal KS. Selective killing of human breast cancer cells by the styryl lactone (R)-goniothalamin is mediated by glutathione conjugation, induction of oxidative stress and marked reactivation of the R175H mutant p53 protein. Carcinogenesis 2018; 39(11):1399-410. doi: 10.1093/carcin/bgy093 [Crossref] [ Google Scholar]
- Nakagawa H, Maeda S. Inflammation- and stress-related signaling pathways in hepatocarcinogenesis. World J Gastroenterol 2012; 18(31):4071-81. doi: 10.3748/wjg.v18.i31.4071 [Crossref] [ Google Scholar]
- Checa J, Aran JM. Reactive oxygen species: drivers of physiological and pathological processes. J Inflamm Res 2020; 13:1057-73. doi: 10.2147/jir.S275595 [Crossref] [ Google Scholar]
- Jacquemin G, Margiotta D, Kasahara A, Bassoy EY, Walch M, Thiery J. Granzyme B-induced mitochondrial ROS are required for apoptosis. Cell Death Differ 2015; 22(5):862-74. doi: 10.1038/cdd.2014.180 [Crossref] [ Google Scholar]
- Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu Y. ROS and ROS-mediated cellular signaling. Oxid Med Cell Longev 2016; 2016:4350965. doi: 10.1155/2016/4350965 [Crossref] [ Google Scholar]
- Son Y, Cheong YK, Kim NH, Chung HT, Kang DG, Pae HO. Mitogen-activated protein kinases and reactive oxygen species: how can ROS activate MAPK Pathways?. J Signal Transduct 2011; 2011:792639. doi: 10.1155/2011/792639 [Crossref] [ Google Scholar]
- McCubrey JA, Lahair MM, Franklin RA. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid Redox Signal 2006; 8(9-10):1775-89. doi: 10.1089/ars.2006.8.1775 [Crossref] [ Google Scholar]
- Warnatsch A, Tsourouktsoglou TD, Branzk N, Wang Q, Reincke S, Herbst S. Reactive oxygen species localization programs inflammation to clear microbes of different size. Immunity 2017; 46(3):421-32. doi: 10.1016/j.immuni.2017.02.013 [Crossref] [ Google Scholar]
- Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 2007; 26(22):3291-310. doi: 10.1038/sj.onc.1210422 [Crossref] [ Google Scholar]
- McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta 2007; 1773(8):1263-84. doi: 10.1016/j.bbamcr.2006.10.001 [Crossref] [ Google Scholar]
- Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene 2007; 26(22):3279-90. doi: 10.1038/sj.onc.1210421 [Crossref] [ Google Scholar]
- Yang S, Liu G. Targeting the Ras/Raf/MEK/ERK pathway in hepatocellular carcinoma. Oncol Lett 2017; 13(3):1041-7. doi: 10.3892/ol.2017.5557 [Crossref] [ Google Scholar]
- He Y, Sun MM, Zhang GG, Yang J, Chen KS, Xu WW. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct Target Ther 2021; 6(1):425. doi: 10.1038/s41392-021-00828-5 [Crossref] [ Google Scholar]
- Asati V, Mahapatra DK, Bharti SK. PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signaling pathways inhibitors as anticancer agents: Structural and pharmacological perspectives. Eur J Med Chem 2016; 109:314-41. doi: 10.1016/j.ejmech.2016.01.012 [Crossref] [ Google Scholar]
- Steelman LS, Abrams SL, Whelan J, Bertrand FE, Ludwig DE, Bäsecke J. Contributions of the Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways to leukemia. Leukemia 2008; 22(4):686-707. doi: 10.1038/leu.2008.26 [Crossref] [ Google Scholar]
- Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov 2014; 13(2):140-56. doi: 10.1038/nrd4204 [Crossref] [ Google Scholar]
- Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y, Hu LL. ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med 2020; 19(3):1997-2007. doi: 10.3892/etm.2020.8454 [Crossref] [ Google Scholar]
- Deschênes-Simard X, Gaumont-Leclerc MF, Bourdeau V, Lessard F, Moiseeva O, Forest V. Tumor suppressor activity of the ERK/MAPK pathway by promoting selective protein degradation. Genes Dev 2013; 27(8):900-15. doi: 10.1101/gad.203984.112 [Crossref] [ Google Scholar]
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 1997; 88(5):593-602. doi: 10.1016/s0092-8674(00)81902-9 [Crossref] [ Google Scholar]
- Schmitt CA, Wang B, Demaria M. Senescence and cancer - role and therapeutic opportunities. Nat Rev Clin Oncol 2022; 19(10):619-36. doi: 10.1038/s41571-022-00668-4 [Crossref] [ Google Scholar]
- Lau L, David G. Pro- and anti-tumorigenic functions of the senescence-associated secretory phenotype. Expert Opin Ther Targets 2019; 23(12):1041-51. doi: 10.1080/14728222.2019.1565658 [Crossref] [ Google Scholar]
- Leelahavanichkul K, Amornphimoltham P, Molinolo AA, Basile JR, Koontongkaew S, Gutkind JS. A role for p38 MAPK in head and neck cancer cell growth and tumor-induced angiogenesis and lymphangiogenesis. Mol Oncol 2014; 8(1):105-18. doi: 10.1016/j.molonc.2013.10.003 [Crossref] [ Google Scholar]
- Burotto M, Chiou VL, Lee JM, Kohn EC. The MAPK pathway across different malignancies: a new perspective. Cancer 2014; 120(22):3446-56. doi: 10.1002/cncr.28864 [Crossref] [ Google Scholar]
- Zuo L, Prather ER, Stetskiv M, Garrison DE, Meade JR, Peace TI. Inflammaging and oxidative stress in human diseases: from molecular mechanisms to novel treatments. Int J Mol Sci 2019; 20(18):4472. doi: 10.3390/ijms20184472 [Crossref] [ Google Scholar]
- Liu Y, Zeng G. Cancer and innate immune system interactions: translational potentials for cancer immunotherapy. J Immunother 2012; 35(4):299-308. doi: 10.1097/CJI.0b013e3182518e83 [Crossref] [ Google Scholar]
- Ponomarev AV, Shubina IZ. Insights into mechanisms of tumor and immune system interaction: association with wound healing. Front Oncol 2019; 9:1115. doi: 10.3389/fonc.2019.01115 [Crossref] [ Google Scholar]
- Riise RE, Bernson E, Aurelius J, Martner A, Pesce S, Della Chiesa M. TLR-stimulated neutrophils instruct NK cells to trigger dendritic cell maturation and promote adaptive T cell responses. J Immunol 2015; 195(3):1121-8. doi: 10.4049/jimmunol.1500709 [Crossref] [ Google Scholar]
- Kerbel RS. Tumor angiogenesis. N Engl J Med 2008; 358(19):2039-49. doi: 10.1056/NEJMra0706596 [Crossref] [ Google Scholar]
- Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol 2007; 127(3):514-25. doi: 10.1038/sj.jid.5700701 [Crossref] [ Google Scholar]
- Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol 2010; 10(12):826-37. doi: 10.1038/nri2873 [Crossref] [ Google Scholar]
- Chang RB, Beatty GL. The interplay between innate and adaptive immunity in cancer shapes the productivity of cancer immunosurveillance. J Leukoc Biol 2020; 108(1):363-76. doi: 10.1002/jlb.3mir0320-475r [Crossref] [ Google Scholar]
- Montironi C, Muñoz-Pinedo C, Eldering E. Hematopoietic versus solid cancers and T cell dysfunction: looking for similarities and distinctions. Cancers (Basel) 2021; 13(2):284. doi: 10.3390/cancers13020284 [Crossref] [ Google Scholar]
- Markov OV, Mironova NL, Vlasov VV, Zenkova MA. Molecular and cellular mechanisms of antitumor immune response activation by dendritic cells. Acta Naturae 2016; 8(3):17-30. [ Google Scholar]
- Jacqueline C, Bourfia Y, Hbid H, Sorci G, Thomas F, Roche B. Interactions between immune challenges and cancer cells proliferation: timing does matter!. Evol Med Public Health 2016; 2016(1):299-311. doi: 10.1093/emph/eow025 [Crossref] [ Google Scholar]
- Calì B, Molon B, Viola A. Tuning cancer fate: the unremitting role of host immunity. Open Biol 2017; 7(4):170006. doi: 10.1098/rsob.170006 [Crossref] [ Google Scholar]
- Triantafilou M, Lepper PM, Olden R, Dias IS, Triantafilou K. Location, location, location: is membrane partitioning everything when it comes to innate immune activation?. Mediators Inflamm 2011; 2011:186093. doi: 10.1155/2011/186093 [Crossref] [ Google Scholar]
- Morán GA, Parra-Medina R, Cardona AG, Quintero-Ronderos P, Rodríguez ÉG. Cytokines, chemokines and growth factors. In: Autoimmunity: From Bench to Bedside [Internet]. Bogota, Colombia: El Rosario University Press; 2013.
- Turner MD, Nedjai B, Hurst T, Pennington DJ. Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta 2014; 1843(11):2563-82. doi: 10.1016/j.bbamcr.2014.05.014 [Crossref] [ Google Scholar]
- Barnes TC, Anderson ME, Edwards SW, Moots RJ. Neutrophil-derived reactive oxygen species in SSc. Rheumatology (Oxford) 2012; 51(7):1166-9. doi: 10.1093/rheumatology/ker520 [Crossref] [ Google Scholar]
- Yan B, Han P, Pan L, Lu W, Xiong J, Zhang M. IL-1β and reactive oxygen species differentially regulate neutrophil directional migration and Basal random motility in a zebrafish injury-induced inflammation model. J Immunol 2014; 192(12):5998-6008. doi: 10.4049/jimmunol.1301645 [Crossref] [ Google Scholar]
- Mantovani A. Macrophages, neutrophils, and cancer: a double-edged sword. New J Sci 2014; 2014(1):271940. doi: 10.1155/2014/271940 [Crossref] [ Google Scholar]
- Peake J, Suzuki K. Neutrophil activation, antioxidant supplements and exercise-induced oxidative stress. Exerc Immunol Rev 2004; 10:129-41. [ Google Scholar]
- Wang X, Qiu L, Li Z, Wang XY, Yi H. Understanding the multifaceted role of neutrophils in cancer and autoimmune diseases. Front Immunol 2018; 9:2456. doi: 10.3389/fimmu.2018.02456 [Crossref] [ Google Scholar]
- Mukaida N, Sasaki SI, Baba T. Two-faced roles of tumor-associated neutrophils in cancer development and progression. Int J Mol Sci 2020; 21(10):3457. doi: 10.3390/ijms21103457 [Crossref] [ Google Scholar]
- Wright HL, Moots RJ, Bucknall RC, Edwards SW. Neutrophil function in inflammation and inflammatory diseases. Rheumatology (Oxford) 2010; 49(9):1618-31. doi: 10.1093/rheumatology/keq045 [Crossref] [ Google Scholar]
- Austermann J, Roth J, Barczyk-Kahlert K. The good and the bad: monocytes’ and macrophages’ diverse functions in inflammation. Cells 2022; 11(12):1979. doi: 10.3390/cells11121979 [Crossref] [ Google Scholar]
- Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer 2016; 16(7):431-46. doi: 10.1038/nrc.2016.52 [Crossref] [ Google Scholar]
- Selders GS, Fetz AE, Radic MZ, Bowlin GL. An overview of the role of neutrophils in innate immunity, inflammation and host-biomaterial integration. Regen Biomater 2017; 4(1):55-68. doi: 10.1093/rb/rbw041 [Crossref] [ Google Scholar]
- Kruger P, Saffarzadeh M, Weber AN, Rieber N, Radsak M, von Bernuth H. Neutrophils: between host defence, immune modulation, and tissue injury. PLoS Pathog 2015; 11(3):e1004651. doi: 10.1371/journal.ppat.1004651 [Crossref] [ Google Scholar]
- Shaul ME, Fridlender ZG. Neutrophils as active regulators of the immune system in the tumor microenvironment. J Leukoc Biol 2017; 102(2):343-9. doi: 10.1189/jlb.5MR1216-508R [Crossref] [ Google Scholar]
- Tan HY, Wang N, Li S, Hong M, Wang X, Feng Y. The reactive oxygen species in macrophage polarization: reflecting its dual role in progression and treatment of human diseases. Oxid Med Cell Longev 2016; 2016:2795090. doi: 10.1155/2016/2795090 [Crossref] [ Google Scholar]
- Herb M, Schramm M. Functions of ROS in macrophages and antimicrobial immunity. Antioxidants (Basel) 2021; 10(2):313. doi: 10.3390/antiox10020313 [Crossref] [ Google Scholar]
- Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol 2000; 164(12):6166-73. doi: 10.4049/jimmunol.164.12.6166 [Crossref] [ Google Scholar]
- Hirayama D, Iida T, Nakase H. The phagocytic function of macrophage-enforcing innate immunity and tissue homeostasis. Int J Mol Sci 2017; 19(1):92. doi: 10.3390/ijms19010092 [Crossref] [ Google Scholar]
- Dandekar RC, Kingaonkar AV, Dhabekar GS. Role of macrophages in malignancy. Ann Maxillofac Surg 2011; 1(2):150-4. doi: 10.4103/2231-0746.92782 [Crossref] [ Google Scholar]
- Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 2010; 11(10):889-96. doi: 10.1038/ni.1937 [Crossref] [ Google Scholar]
- Huang SC, Everts B, Ivanova Y, O’Sullivan D, Nascimento M, Smith AM. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat Immunol 2014; 15(9):846-55. doi: 10.1038/ni.2956 [Crossref] [ Google Scholar]
- Arora S, Dev K, Agarwal B, Das P, Syed MA. Macrophages: their role, activation and polarization in pulmonary diseases. Immunobiology 2018; 223(4-5):383-96. doi: 10.1016/j.imbio.2017.11.001 [Crossref] [ Google Scholar]
- Fujii J, Osaki T. Involvement of nitric oxide in protecting against radical species and autoregulation of M1-polarized macrophages through metabolic remodeling. Molecules 2023; 28(2):814. doi: 10.3390/molecules28020814 [Crossref] [ Google Scholar]
- Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity 2010; 32(5):593-604. doi: 10.1016/j.immuni.2010.05.007 [Crossref] [ Google Scholar]
- Sionov RV, Fridlender ZG, Granot Z. The multifaceted roles neutrophils play in the tumor microenvironment. Cancer Microenviron 2015; 8(3):125-58. doi: 10.1007/s12307-014-0147-5 [Crossref] [ Google Scholar]
- Richards MK, Liu F, Iwasaki H, Akashi K, Link DC. Pivotal role of granulocyte colony-stimulating factor in the development of progenitors in the common myeloid pathway. Blood 2003; 102(10):3562-8. doi: 10.1182/blood-2003-02-0593 [Crossref] [ Google Scholar]
- Masucci MT, Minopoli M, Carriero MV. Tumor associated neutrophils Their role in tumorigenesis, metastasis, prognosis and therapy. Front Oncol 2019; 9:1146. doi: 10.3389/fonc.2019.01146 [Crossref] [ Google Scholar]
- DiCamillo SJ, Yang S, Panchenko MV, Toselli PA, Naggar EF, Rich CB. Neutrophil elastase-initiated EGFR/MEK/ERK signaling counteracts stabilizing effect of autocrine TGF-beta on tropoelastin mRNA in lung fibroblasts. Am J Physiol Lung Cell Mol Physiol 2006; 291(2):L232-43. doi: 10.1152/ajplung.00530.2005 [Crossref] [ Google Scholar]
- Yang R, Zhong L, Yang XQ, Jiang KL, Li L, Song H. Neutrophil elastase enhances the proliferation and decreases apoptosis of leukemia cells via activation of PI3K/Akt signaling. Mol Med Rep 2016; 13(5):4175-82. doi: 10.3892/mmr.2016.5051 [Crossref] [ Google Scholar]
- Wada Y, Yoshida K, Hihara J, Konishi K, Tanabe K, Ukon K. Sivelestat, a specific neutrophil elastase inhibitor, suppresses the growth of gastric carcinoma cells by preventing the release of transforming growth factor-alpha. Cancer Sci 2006; 97(10):1037-43. doi: 10.1111/j.1349-7006.2006.00278.x [Crossref] [ Google Scholar]
- Nathan C, Cunningham-Bussel A. Beyond oxidative stress: an immunologist’s guide to reactive oxygen species. Nat Rev Immunol 2013; 13(5):349-61. doi: 10.1038/nri3423 [Crossref] [ Google Scholar]
- San José G, Bidegain J, Robador PA, Díez J, Fortuño A, Zalba G. Insulin-induced NADPH oxidase activation promotes proliferation and matrix metalloproteinase activation in monocytes/macrophages. Free Radic Biol Med 2009; 46(8):1058-67. doi: 10.1016/j.freeradbiomed.2009.01.009 [Crossref] [ Google Scholar]
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS. Neutrophil extracellular traps kill bacteria. Science 2004; 303(5663):1532-5. doi: 10.1126/science.1092385 [Crossref] [ Google Scholar]
- Liao Z, Chua D, Tan NS. Reactive oxygen species: a volatile driver of field cancerization and metastasis. Mol Cancer 2019; 18(1):65. doi: 10.1186/s12943-019-0961-y [Crossref] [ Google Scholar]
- Harbort CJ, Soeiro-Pereira PV, von Bernuth H, Kaindl AM, Costa-Carvalho BT, Condino-Neto A. Neutrophil oxidative burst activates ATM to regulate cytokine production and apoptosis. Blood 2015; 126(26):2842-51. doi: 10.1182/blood-2015-05-645424 [Crossref] [ Google Scholar]
- Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity 2014; 41(1):49-61. doi: 10.1016/j.immuni.2014.06.010 [Crossref] [ Google Scholar]
- Kim J, Bae JS. Tumor-associated macrophages and neutrophils in tumor microenvironment. Mediators Inflamm 2016; 2016:6058147. doi: 10.1155/2016/6058147 [Crossref] [ Google Scholar]
- Wu L, Zhang XH. Tumor-associated neutrophils and macrophages-heterogenous but not chaotic. Front Immunol 2020; 11:553967. doi: 10.3389/fimmu.2020.553967 [Crossref] [ Google Scholar]
- Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 2009; 16(3):183-94. doi: 10.1016/j.ccr.2009.06.017 [Crossref] [ Google Scholar]
- Winterbourn CC, Kettle AJ, Hampton MB. Reactive oxygen species and neutrophil function. Annu Rev Biochem 2016; 85:765-92. doi: 10.1146/annurev-biochem-060815-014442 [Crossref] [ Google Scholar]
- Cadet J, Wagner JR. DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation. Cold Spring Harb Perspect Biol 2013; 5(2):a012559. doi: 10.1101/cshperspect.a012559 [Crossref] [ Google Scholar]
- Cooke MS, Evans MD, Dizdaroglu M, Lunec J. Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J 2003; 17(10):1195-214. doi: 10.1096/fj.02-0752rev [Crossref] [ Google Scholar]
- Uribe-Querol E, Rosales C. Neutrophils in cancer: two sides of the same coin. J Immunol Res 2015; 2015:983698. doi: 10.1155/2015/983698 [Crossref] [ Google Scholar]
- Thompson PA, Khatami M, Baglole CJ, Sun J, Harris SA, Moon EY. Environmental immune disruptors, inflammation and cancer risk. Carcinogenesis 2015; 36(Suppl 1):S232-53. doi: 10.1093/carcin/bgv038 [Crossref] [ Google Scholar]
- Cronkite DA, Strutt TM. The regulation of inflammation by innate and adaptive lymphocytes. J Immunol Res 2018; 2018:1467538. doi: 10.1155/2018/1467538 [Crossref] [ Google Scholar]
- Fiuza C, Suffredini AF. Human models of innate immunity: local and systemic inflammatory responses. J Endotoxin Res 2001; 7(5):385-8. [ Google Scholar]
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2002; 2(3):161-74. doi: 10.1038/nrc745 [Crossref] [ Google Scholar]
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Lymphocytes and the cellular basis of adaptive immunity. In: Molecular Biology of the Cell. 4th ed. New York: Garland Science; 2002.
- Junt T, Scandella E, Ludewig B. Form follows function: lymphoid tissue microarchitecture in antimicrobial immune defence. Nat Rev Immunol 2008; 8(10):764-75. doi: 10.1038/nri2414 [Crossref] [ Google Scholar]
- Pennock ND, White JT, Cross EW, Cheney EE, Tamburini BA, Kedl RM. T cell responses: naive to memory and everything in between. Adv Physiol Educ 2013; 37(4):273-83. doi: 10.1152/advan.00066.2013 [Crossref] [ Google Scholar]
- Chen J, Ge Q, Cho B, Eise HN. T cell proliferation, differentiation, and restoration in lymphopenic individuals: for contributed volumes. Adv Exp Med Biol 2002; 512:135-9. doi: 10.1007/978-1-4615-0757-4_18 [Crossref] [ Google Scholar]
- Takeuchi Y, Nishikawa H. Roles of regulatory T cells in cancer immunity. Int Immunol 2016; 28(8):401-9. doi: 10.1093/intimm/dxw025 [Crossref] [ Google Scholar]
- Trujillo JA, Sweis RF, Bao R, Luke JJ. T cell-inflamed versus non-T cell-inflamed tumors: a conceptual framework for cancer immunotherapy drug development and combination therapy selection. Cancer Immunol Res 2018; 6(9):990-1000. doi: 10.1158/2326-6066.Cir-18-0277 [Crossref] [ Google Scholar]
- Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood 2008; 112(5):1557-69. doi: 10.1182/blood-2008-05-078154 [Crossref] [ Google Scholar]
- Zhu J, Paul WE. Peripheral CD4 + T-cell differentiation regulated by networks of cytokines and transcription factors. Immunol Rev 2010; 238(1):247-62. doi: 10.1111/j.1600-065X.2010.00951.x [Crossref] [ Google Scholar]
- O’Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4 + T cells. Science 2010; 327(5969):1098-102. doi: 10.1126/science.1178334 [Crossref] [ Google Scholar]
- Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest 2007; 117(5):1175-83. doi: 10.1172/jci31537 [Crossref] [ Google Scholar]
- Habanjar O, Bingula R, Decombat C, Diab-Assaf M, Caldefie-Chezet F, Delort L. Crosstalk of inflammatory cytokines within the breast tumor microenvironment. Int J Mol Sci 2023; 24(4):4002. doi: 10.3390/ijms24044002 [Crossref] [ Google Scholar]
- Kesarwani P, Murali AK, Al-Khami AA, Mehrotra S. Redox regulation of T-cell function: from molecular mechanisms to significance in human health and disease. Antioxid Redox Signal 2013; 18(12):1497-534. doi: 10.1089/ars.2011.4073 [Crossref] [ Google Scholar]
- Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O’Garra A, Murphy KM. Development of Th1 CD4 + T cells through IL-12 produced by Listeria-induced macrophages. Science 1993; 260(5107):547-9. doi: 10.1126/science.8097338 [Crossref] [ Google Scholar]
- Lighvani AA, Frucht DM, Jankovic D, Yamane H, Aliberti J, Hissong BD. T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells. Proc Natl Acad Sci U S A 2001; 98(26):15137-42. doi: 10.1073/pnas.261570598 [Crossref] [ Google Scholar]
- Bao K, Reinhardt RL. The differential expression of IL-4 and IL-13 and its impact on type-2 immunity. Cytokine 2015; 75(1):25-37. doi: 10.1016/j.cyto.2015.05.008 [Crossref] [ Google Scholar]
- Zhu J. T helper 2 (Th2) cell differentiation, type 2 innate lymphoid cell (ILC2) development and regulation of interleukin-4 (IL-4) and IL-13 production. Cytokine 2015; 75(1):14-24. doi: 10.1016/j.cyto.2015.05.010 [Crossref] [ Google Scholar]
- Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol 2014; 6(10):a016295. doi: 10.1101/cshperspect.a016295 [Crossref] [ Google Scholar]
- Goldman JL, Sammani S, Kempf C, Saadat L, Letsiou E, Wang T. Pleiotropic effects of interleukin-6 in a “two-hit” murine model of acute respiratory distress syndrome. Pulm Circ 2014; 4(2):280-8. doi: 10.1086/675991 [Crossref] [ Google Scholar]
- Abbas AK. The surprising story of IL-2: from experimental models to clinical application. Am J Pathol 2020; 190(9):1776-81. doi: 10.1016/j.ajpath.2020.05.007 [Crossref] [ Google Scholar]
- Escobar J, Pereda J, Arduini A, Sandoval J, Sabater L, Aparisi L. Cross-talk between oxidative stress and pro-inflammatory cytokines in acute pancreatitis: a key role for protein phosphatases. Curr Pharm Des 2009; 15(26):3027-42. doi: 10.2174/138161209789058075 [Crossref] [ Google Scholar]
- Elmarakby AA, Sullivan JC. Relationship between oxidative stress and inflammatory cytokines in diabetic nephropathy. Cardiovasc Ther 2012; 30(1):49-59. doi: 10.1111/j.1755-5922.2010.00218.x [Crossref] [ Google Scholar]
- di Penta A, Moreno B, Reix S, Fernandez-Diez B, Villanueva M, Errea O. Oxidative stress and proinflammatory cytokines contribute to demyelination and axonal damage in a cerebellar culture model of neuroinflammation. PLoS One 2013; 8(2):e54722. doi: 10.1371/journal.pone.0054722 [Crossref] [ Google Scholar]
- Pereda J, Sabater L, Aparisi L, Escobar J, Sandoval J, Viña J. Interaction between cytokines and oxidative stress in acute pancreatitis. Curr Med Chem 2006; 13(23):2775-87. doi: 10.2174/092986706778522011 [Crossref] [ Google Scholar]
- Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol 2002; 20:55-72. doi: 10.1146/annurev.immunol.20.091301.131133 [Crossref] [ Google Scholar]
- Rahman I, MacNee W. Role of transcription factors in inflammatory lung diseases. Thorax 1998; 53(7):601-12. doi: 10.1136/thx.53.7.601 [Crossref] [ Google Scholar]
- Meyer M, Schreck R, Baeuerle PA. H2O2 and antioxidants have opposite effects on activation of NF-κB and AP-1 in intact cells: AP-1 as secondary antioxidant-responsive factor. EMBO J 1993; 12(5):2005-15. doi: 10.1002/j.1460-2075.1993.tb05850.x [Crossref] [ Google Scholar]
- Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-κB transcription factor and HIV-1. EMBO J 1991; 10(8):2247-58. doi: 10.1002/j.1460-2075.1991.tb07761.x [Crossref] [ Google Scholar]
- Parameswaran N, Patial S. Tumor necrosis factor-α signaling in macrophages. Crit Rev Eukaryot Gene Expr 2010; 20(2):87-103. doi: 10.1615/critreveukargeneexpr.v20.i2.10 [Crossref] [ Google Scholar]
- Sun SC, Ley SC. New insights into NF-κB regulation and function. Trends Immunol 2008; 29(10):469-78. doi: 10.1016/j.it.2008.07.003 [Crossref] [ Google Scholar]
- Garg AK, Aggarwal BB. Reactive oxygen intermediates in TNF signaling. Mol Immunol 2002; 39(9):509-17. doi: 10.1016/s0161-5890(02)00207-9 [Crossref] [ Google Scholar]
- Dabrowski A, Boguslowicz C, Dabrowska M, Tribillo I, Gabryelewicz A. Reactive oxygen species activate mitogen-activated protein kinases in pancreatic acinar cells. Pancreas 2000; 21(4):376-84. doi: 10.1097/00006676-200011000-00008 [Crossref] [ Google Scholar]
- Samuel I, Zaheer A, Fisher RA. In vitro evidence for role of ERK, p38, and JNK in exocrine pancreatic cytokine production. J Gastrointest Surg 2006; 10(10):1376-83. doi: 10.1016/j.gassur.2006.09.003 [Crossref] [ Google Scholar]
- Figueroa YG, Chan AK, Ibrahim R, Tang Y, Burow ME, Alam J. NF-κB plays a key role in hypoxia-inducible factor-1-regulated erythropoietin gene expression. Exp Hematol 2002; 30(12):1419-27. doi: 10.1016/s0301-472x(02)00934-7 [Crossref] [ Google Scholar]
- Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V. NF-κB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature 2008; 453(7196):807-11. doi: 10.1038/nature06905 [Crossref] [ Google Scholar]
- Schroder K, Tschopp J. The inflammasomes. Cell 2010; 140(6):821-32. doi: 10.1016/j.cell.2010.01.040 [Crossref] [ Google Scholar]
- Yilmaz Ö, Lee KL. The inflammasome and danger molecule signaling: at the crossroads of inflammation and pathogen persistence in the oral cavity. Periodontol 2000 2015; 69(1):83-95. doi: 10.1111/prd.12084 [Crossref] [ Google Scholar]
- Miao EA, Rajan JV, Aderem A. Caspase-1-induced pyroptotic cell death. Immunol Rev 2011; 243(1):206-14. doi: 10.1111/j.1600-065X.2011.01044.x [Crossref] [ Google Scholar]
- Abderrazak A, Syrovets T, Couchie D, El Hadri K, Friguet B, Simmet T. NLRP3 inflammasome: from a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases. Redox Biol 2015; 4:296-307. doi: 10.1016/j.redox.2015.01.008 [Crossref] [ Google Scholar]
- Abais JM, Xia M, Zhang Y, Boini KM, Li PL. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector?. Antioxid Redox Signal 2015; 22(13):1111-29. doi: 10.1089/ars.2014.5994 [Crossref] [ Google Scholar]
- Kabe Y, Ando K, Hirao S, Yoshida M, Handa H. Redox regulation of NF-κB activation: distinct redox regulation between the cytoplasm and the nucleus. Antioxid Redox Signal 2005; 7(3-4):395-403. doi: 10.1089/ars.2005.7.395 [Crossref] [ Google Scholar]
- Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D. Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol 2009; 183(2):787-91. doi: 10.4049/jimmunol.0901363 [Crossref] [ Google Scholar]
- Rahal A, Kumar A, Singh V, Yadav B, Tiwari R, Chakraborty S. Oxidative stress, prooxidants, and antioxidants: the interplay. Biomed Res Int 2014; 2014:761264. doi: 10.1155/2014/761264 [Crossref] [ Google Scholar]
- Li G, Ding K, Qiao Y, Zhang L, Zheng L, Pan T. Flavonoids regulate inflammation and oxidative stress in cancer. Molecules 2020; 25(23):5628. doi: 10.3390/molecules25235628 [Crossref] [ Google Scholar]
- John AS, Ankem MK, Damodaran C. Oxidative stress: a promising target for chemoprevention. Curr Pharmacol Rep 2016; 2(2):73-81. doi: 10.1007/s40495-016-0052-3 [Crossref] [ Google Scholar]
- Conde de la Rosa L, Schoemaker MH, Vrenken TE, Buist-Homan M, Havinga R, Jansen PL. Superoxide anions and hydrogen peroxide induce hepatocyte death by different mechanisms: involvement of JNK and ERK MAP kinases. J Hepatol 2006; 44(5):918-29. doi: 10.1016/j.jhep.2005.07.034 [Crossref] [ Google Scholar]
- Chandra J, Samali A, Orrenius S. Triggering and modulation of apoptosis by oxidative stress. Free Radic Biol Med 2000; 29(3-4):323-33. doi: 10.1016/s0891-5849(00)00302-6 [Crossref] [ Google Scholar]
- Kumari S, Badana AK, G MM, G S, Malla R. Reactive oxygen species: a key constituent in cancer survival. Biomark Insights 2018; 13:1177271918755391. doi: 10.1177/1177271918755391 [Crossref] [ Google Scholar]
- Nagamachi M, Sakata D, Kabashima K, Furuyashiki T, Murata T, Segi-Nishida E. Facilitation of Th1-mediated immune response by prostaglandin E receptor EP1. J Exp Med 2007; 204(12):2865-74. doi: 10.1084/jem.20070773 [Crossref] [ Google Scholar]
- Konidena A, Sharma S, Patil DJ, Dixit A, Gupta R, Kaur M. Immunosuppressants in oral medicine: a review. J Indian Acad Oral Med Radiol 2017; 29(4):306-13. doi: 10.4103/jiaomr.JIAOMR_24_17 [Crossref] [ Google Scholar]
- Bascones-Martinez A, Mattila R, Gomez-Font R, Meurman JH. Immunomodulatory drugs: oral and systemic adverse effects. Med Oral Patol Oral Cir Bucal 2014; 19(1):e24-31. doi: 10.4317/medoral.19087 [Crossref] [ Google Scholar]
- Ramamoorthy S, Cidlowski JA. Corticosteroids: mechanisms of action in health and disease. Rheum Dis Clin North Am 2016; 42(1):15-31. doi: 10.1016/j.rdc.2015.08.002 [Crossref] [ Google Scholar]
- Barnes PJ. Mechanisms and resistance in glucocorticoid control of inflammation. J Steroid Biochem Mol Biol 2010; 120(2-3):76-85. doi: 10.1016/j.jsbmb.2010.02.018 [Crossref] [ Google Scholar]
- Cavalli G, Dinarello CA. Anakinra therapy for non-cancer inflammatory diseases. Front Pharmacol 2018; 9:1157. doi: 10.3389/fphar.2018.01157 [Crossref] [ Google Scholar]
- Cohan SL, Lucassen EB, Romba MC, Linch SN. Daclizumab: mechanisms of action, therapeutic efficacy, adverse events and its uncovering the potential role of innate immune system recruitment as a treatment strategy for relapsing multiple sclerosis. Biomedicines 2019; 7(1):18. doi: 10.3390/biomedicines7010018 [Crossref] [ Google Scholar]
- Sebba A. Tocilizumab: the first interleukin-6-receptor inhibitor. Am J Health Syst Pharm 2008; 65(15):1413-8. doi: 10.2146/ajhp070449 [Crossref] [ Google Scholar]
- Davis JC Jr, Van Der Heijde D, Braun J, Dougados M, Cush J, Clegg DO. Recombinant human tumor necrosis factor receptor (etanercept) for treating ankylosing spondylitis: a randomized, controlled trial. Arthritis Rheum 2003; 48(11):3230-6. doi: 10.1002/art.11325 [Crossref] [ Google Scholar]
- Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther 2008; 117(2):244-79. doi: 10.1016/j.pharmthera.2007.10.001 [Crossref] [ Google Scholar]
- Rutgeerts P, Van Assche G, Vermeire S. Review article: Infliximab therapy for inflammatory bowel disease--seven years on. Aliment Pharmacol Ther 2006; 23(4):451-63. doi: 10.1111/j.1365-2036.2006.02786.x [Crossref] [ Google Scholar]
- Bang LM, Keating GM. Adalimumab: a review of its use in rheumatoid arthritis. BioDrugs 2004; 18(2):121-39. doi: 10.2165/00063030-200418020-00005 [Crossref] [ Google Scholar]
- Ogino MH, Tadi P. Cyclophosphamide. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2020.
- Mohammadi O, Kassim TA. Azathioprine. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2019.
- Ladrière M. [Current indications of azathioprine in nephrology]. Nephrol Ther 2013; 9(1):8-12. doi: 10.1016/j.nephro.2012.08.002.[French] [Crossref] [ Google Scholar]
- Cronstein BN, Aune TM. Methotrexate and its mechanisms of action in inflammatory arthritis. Nat Rev Rheumatol 2020; 16(3):145-54. doi: 10.1038/s41584-020-0373-9 [Crossref] [ Google Scholar]
- Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology 2000; 47(2-3):85-118. doi: 10.1016/s0162-3109(00)00188-0 [Crossref] [ Google Scholar]
- Allison AC, Eugui EM. Purine metabolism and immunosuppressive effects of mycophenolate mofetil (MMF). Clin Transplant 1996; 10(1 Pt 2):77-84. [ Google Scholar]
- Mohty M. Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia 2007; 21(7):1387-94. doi: 10.1038/sj.leu.2404683 [Crossref] [ Google Scholar]
- Bonnefoy-Berard N, Genestier L, Preville X, Revillard JP. TNFα and CD95-L contribute to apoptosis of activated lymphocytes triggered by ATGs. Transplant Proc 1999; 31(1-2):775-7. doi: 10.1016/s0041-1345(98)02100-9 [Crossref] [ Google Scholar]
- Genestier L, Fournel S, Flacher M, Assossou O, Revillard JP, Bonnefoy-Berard N. Induction of Fas (Apo-1, CD95)-mediated apoptosis of activated lymphocytes by polyclonal antithymocyte globulins. Blood 1998; 91(7):2360-8. [ Google Scholar]
- Norman DJ. Mechanisms of action and overview of OKT3. Ther Drug Monit 1995; 17(6):615-20. doi: 10.1097/00007691-199512000-00012 [Crossref] [ Google Scholar]
- Ruck T, Bittner S, Wiendl H, Meuth SG. Alemtuzumab in multiple sclerosis: mechanism of action and beyond. Int J Mol Sci 2015; 16(7):16414-39. doi: 10.3390/ijms160716414 [Crossref] [ Google Scholar]
- Weiner GJ. Rituximab: mechanism of action. Semin Hematol 2010; 47(2):115-23. doi: 10.1053/j.seminhematol.2010.01.011 [Crossref] [ Google Scholar]
- Overall CM, López-Otín C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer 2002; 2(9):657-72. doi: 10.1038/nrc884 [Crossref] [ Google Scholar]
- Vincenzi B, Schiavon G, Silletta M, Santini D, Tonini G. The biological properties of cetuximab. Crit Rev Oncol Hematol 2008; 68(2):93-106. doi: 10.1016/j.critrevonc.2008.07.006 [Crossref] [ Google Scholar]
- Gordon KB, Vaishnaw AK, O’Gorman J, Haney J, Menter A. Treatment of psoriasis with alefacept: correlation of clinical improvement with reductions of memory T-cell counts. Arch Dermatol 2003; 139(12):1563-70. doi: 10.1001/archderm.139.12.1563 [Crossref] [ Google Scholar]
- Bonelli M, Scheinecker C. How does abatacept really work in rheumatoid arthritis?. Curr Opin Rheumatol 2018; 30(3):295-300. doi: 10.1097/bor.0000000000000491 [Crossref] [ Google Scholar]
- Herrero-Beaumont G, Martínez Calatrava MJ, Castañeda S. Abatacept mechanism of action: concordance with its clinical profile. Reumatol Clin 2012; 8(2):78-83. doi: 10.1016/j.reuma.2011.08.002 [Crossref] [ Google Scholar]
- Matsuda S, Koyasu S. Mechanisms of action of cyclosporine. Immunopharmacology 2000; 47(2-3):119-25. doi: 10.1016/s0162-3109(00)00192-2 [Crossref] [ Google Scholar]
- Tedesco D, Haragsim L. Cyclosporine: a review. J Transplant 2012; 2012:230386. doi: 10.1155/2012/230386 [Crossref] [ Google Scholar]
- Thomson AW, Bonham CA, Zeevi A. Mode of action of tacrolimus (FK506): molecular and cellular mechanisms. Ther Drug Monit 1995; 17(6):584-91. doi: 10.1097/00007691-199512000-00007 [Crossref] [ Google Scholar]
- Sehgal SN. Sirolimus: its discovery, biological properties, and mechanism of action. Transplant Proc 2003; 35(3 Suppl):7S-14S. doi: 10.1016/s0041-1345(03)00211-2 [Crossref] [ Google Scholar]
- Mahale A, Abhonkar RS. The review on dapsone. Int J Res Publ Rev 2020; 3(11):2955-60. [ Google Scholar]
- Wozel G, Blasum C. Dapsone in dermatology and beyond. Arch Dermatol Res 2014; 306(2):103-24. doi: 10.1007/s00403-013-1409-7 [Crossref] [ Google Scholar]
- Singh K, Bhori M, Kasu YA, Bhat G, Marar T. Antioxidants as precision weapons in war against cancer chemotherapy induced toxicity - exploring the armoury of obscurity. Saudi Pharm J 2018; 26(2):177-90. doi: 10.1016/j.jsps.2017.12.013 [Crossref] [ Google Scholar]
- Ferdous UT, Yusof ZNB. Medicinal prospects of antioxidants from algal sources in cancer therapy. Front Pharmacol 2021; 12:593116. doi: 10.3389/fphar.2021.593116 [Crossref] [ Google Scholar]
- Luo M, Zhou L, Huang Z, Li B, Nice EC, Xu J. Antioxidant therapy in cancer: rationale and progress. Antioxidants (Basel) 2022; 11(6):1128. doi: 10.3390/antiox11061128 [Crossref] [ Google Scholar]
- Calvani M, Pasha A, Favre C. Nutraceutical boom in cancer: inside the labyrinth of reactive oxygen species. Int J Mol Sci 2020; 21(6):1936. doi: 10.3390/ijms21061936 [Crossref] [ Google Scholar]
- Yasueda A, Urushima H, Ito T. Efficacy and interaction of antioxidant supplements as adjuvant therapy in cancer treatment: a systematic review. Integr Cancer Ther 2016; 15(1):17-39. doi: 10.1177/1534735415610427 [Crossref] [ Google Scholar]