Biomed Res Bull. 2(3):108-117.
doi: 10.34172/biomedrb.2024.17
Original Article
The Use of Boron as Semen Extender in Sperm Cryopreservation: A Systematic Review
Anis Sani 1  , Amin Sani 2, Keysan Pourmoghtader 1, Mohammad Hamidi Madani 3, Reza Aletaha 1, Mustafa Numan Bucak 4, *
, Amin Sani 2, Keysan Pourmoghtader 1, Mohammad Hamidi Madani 3, Reza Aletaha 1, Mustafa Numan Bucak 4, *  , Hanieh Salehi-Pourmehr 5, 6, *
, Hanieh Salehi-Pourmehr 5, 6, *  , Sakineh Hajebrahimi 5, 7
, Sakineh Hajebrahimi 5, 7
Author information:
1Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran
2Department of Reproduction and Artificial insemination, Faculty of Veterinary Medicine, Tabriz Medical Sciences, Islamic Azad University, Tabriz, Iran
3Department of Urology, Shahid Labbafinejad Medical Center, Shahid Beheshti University of Medical Sciences, Tehran,Iran
4Faculty of Veterinary Medicine, Selçuk University, Konya, Turkey
5Research Center for Evidence-Based Medicine, Iranian EBM Centre: A JBI Centre of Excellence, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
6Medical Philosophy and History Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
7Urology Department, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Background:
Cryopreservation of human sperm has long been a successful method for managing male fertility and for storing and preserving donor spermatozoa. Boron is one element used in this particular procedure. The primary objective of this study was to conduct a thorough assessment of existing evidence regarding the impact of boron extenders on sperm parameters.
Methods:
The databases PubMed, Scopus, Embase, ProQuest, and the Web of Science were searched up until January 2023 without time or language restrictions, using specified keywords and index terms. The keywords included: (((("reproduction"[MeSH Terms]) OR ("reproduction"[Title/Abstract])) OR ("reproductive"[Title/Abstract])) OR ((("infertility"[MeSH Terms]) OR (infertile*[Title/Abstract])) OR (Sperm [Title/Abstract]))) AND (("boron"[MeSH Terms]) OR (boron [Title/Abstract])). The methodological validity of the quantitative papers selected for inclusion in the systematic review was assessed by two independent reviewers using the standardized ARRIVE Essential 10: Compliance Questionnaire appraisal tools.
Results:
Out of 1602 citations, only five studies met the eligibility criteria to be included in the study. All five studies were conducted in Turkey and used a case-control design with varying samples, including Angora goats, bulls, brown trouts, Merino rams, and Ankara bucks. Freezing semen using boron-added extenders, specifically extenders 1c and 1d, showed the highest motility values in Angora goats. In bulls, different boron doses in extenders were evaluated, with the group containing the lowest boron content exhibiting the best sperm parameters. Incorporating boron into semen extenders improved post-thaw sperm quality, especially in motility. For brown trout sperm, two freezing profiles were tested, revealing that boron improved post-thaw sperm characteristics, with 0.4 mM yielding the best results. In addition, boron supplementation in Merino ram sperm demonstrated that 0.25 mM boron with trehalose improved motility, mitochondrial activity, viability, and acrosome integrity, and reduced DNA damage at 1 mM boron. Moreover, 0.25 and 1 mM boron protected sperm plasma membrane, acrosome integrity, and mitochondrial membrane activity after freeze-thawing, with all boron concentrations offering better cryoprotective benefits than the control group.
Conclusion:
Overall, the findings suggest that boron positively impacts sperm cryopreservation outcomes and could be a valuable addition to semen extenders for enhancing fertility preservation in various animal species.
Keywords: Boron, Semen extender, Sperm cryopreservation, Systematic review
Copyright and License Information
© 2024 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
None.
Introduction
Cryopreservation of human sperm has been a successful method for managing male fertility and storing and preserving donor spermatozoa since it was first developed in the 1960s.1 These procedures are essential to preserve endangered animal species, to breed animals through artificial insemination, and, most importantly, to provide hope for future parenthood for those undergoing treatments or surgeries that may impair gonadal function. Fertility preservation should be made available for autologous use to men before treatments with cytotoxic agents or radiation, vasectomy, social freezing in at-risk patients, and male-to-female transsexual adults and adolescents before initiating hormonal therapies.1-4
The cryopreservation process is complex, involving various procedures, freezing carriers, and cryoprotective agents.5,6 The use of cryopreservation techniques has been shown to increase sperm longevity, but it is important to note that the fertilization capacity of cryopreserved sperm may be hindered due to alterations in sperm’s structural and physiological characteristics.7,8 The freezing process can impact sperm quality after thawing, potentially influenced by various factors such as abrupt temperature fluctuations, osmotic stress, and ice formation.9,10 Furthermore, an enhanced formation of reactive oxygen species (ROS) during the freezing-thawing process can contribute to structural and functional changes in spermatozoa.11,12 Additives are incorporated into the extenders before freezing to mitigate damage and improve sperm characteristics during the freeze-thawing procedure.12-17
Boron is an element utilized in this process.17-19 Despite extensive studies on boron’s toxicity, no harmful effects on the human reproductive system have been discovered thus far.20-23 The World Health Organization (WHO) suggests a daily boron intake of 1–13 mg.24,25 It has been demonstrated that daily supplementation of 100, 200, and 400 mg of boric acid can enhance semen quality in rabbits and benefit their physiological condition.26,27 However, excessive boron intake has been associated with testicular atrophy in male rodents and deleterious effects on reproduction.28-31
A comprehensive examination of boron as a semen extender has not yet been thoroughly explored. Hence, the primary objective of this study was to comprehensively assess existing evidence related to the effects of boron extenders on sperm parameters.
Methods
This systematic review adhered to the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement and the Cochrane Collaboration Handbook.
Eligibility Criteria
Up until January 2023, the databases PubMed, Scopus, Embase, ProQuest, and the Web of Science were searched without time or language restrictions. Additionally, comprehensive backward and forward citation searches were conducted for all included papers. Abstracts, reviews, letters, and theses were excluded from the current study. Studies utilizing nanospheres, nanoribbons, nanoplates, or derivatives of boron nitride were also excluded. Research on the effects of boron as a source on animal sperm parameters, whether beneficial or harmful, was taken into account.
Search Strategy
The objective of the search strategy was to identify and include both published and unpublished studies. A three-step search process was used in this study. After conducting a preliminary limited search in MEDLINE, the text words in the abstract and title were examined. In January 2023, a comprehensive search was performed across all relevant databases using specified keywords and index terms. The keywords were: ((((“reproduction”[MeSH Terms]) OR (“reproduction”[Title/Abstract])) OR (“reproductive”[Title/Abstract])) OR (((“infertility”[MeSH Terms]) OR (infertile*[Title/Abstract])) OR (Sperm [Title/Abstract]))) AND ((“boron”[MeSH Terms]) OR (boron [Title/Abstract])).
The final stage involved a comprehensive review of the reference lists of all the cited papers and articles to identify more relevant studies. This review covered research published on various dates and in different languages.
Data Collection
The PRISMA guidelines were followed to systematically categorize and structure the articles acquired from several search sources. The articles were entered into an Excel spreadsheet. Two researchers independently examined the title and abstract of each article using the inclusion criteria. Subsequently, both researchers individually reviewed the full texts of all studies that successfully passed the initial screening. Any discrepancies between the researchers were resolved through discussion or resorting to a third researcher.
Evaluation of Methodological Quality
Two independent reviewers assessed the methodological validity of the quantitative papers selected for inclusion in the systematic review using standardized ARRIVE Essential 10: Compliance Questionnaire appraisal tools. Potential discrepancies between the reviewers were resolved either through discussion or the involvement of a third reviewer (Table 1).
Table 1.
The Use of Boron as a Semen Extender in Sperm Cryopreservation
|
Author (year)
|
Country
|
Study Design
|
Animal
|
Sample Size
|
Freezing Protocol
|
Thawing Protocol
|
Semen Extender Components
|
|
Study
|
Control
|
| Tripan32 |
Turkey |
Case-control |
Angora goat |
10 |
In + 4°C for 2 hours for equilibration. Frozen in liquid nitrogen vapor and stored in a liquid nitrogen tank (in liquid nitrogen -196 ⁰C). |
In 37°C water bath for 35 seconds |
Boron added extenders: Tris (3.63 g), citric acid (1.82 g), glucose (0.5 g), sodium pentaborate (1a: 0.2,1b: 0.4, 1c: 0.6, 1d: 0.8 g)
extenders with boron instead of glucose: Tris (3.63 g), citric acid (1.82 g), sodium pentaborate (2a: 0.3,2b: 0.5, 2c: 0.7, 2d:0.9 g) |
Tris (3.63 g), citric acid (1.82 g), glucose (0.5 g) |
| Tripan33 |
Turkey |
Case-control |
bull |
4 |
equilibrated at 4 ⁰C for 24 hours. Programmable freezing chamber; decreasing it from 4 ⁰C to the lowest temperature chosen (-140 ⁰C) at 10 ⁰C/minutes. |
30 seconds in a water bath at 37 °C |
1- Tris (3.63 g), citric acid (1.82 g), glucose (0.5 g), Boron (0.4 g) Egg yolk (10%), glycerol (5%)
2- Tris (3.63 g), citric acid (1.82 g), Boron (0.5 g) Egg yolk (10%), glycerol (5%)
3- Tris (3.63 g), citric acid (1.82 g), Boron (0.7 g) Egg yolk (10%), glycerol (5%)
4- Tris (3.63 g), citric acid (1.82 g), Boron (0.9 g) Egg yolk (10%), Glycerol (5%) |
Tris (3.63 g), citric acid (1.82 g), glucose (0.5 g), Egg yolk (10%), glycerol (5%) |
| Bozkurt18 |
Turkey |
Case-control |
Brown trout |
12 |
Programmable freezer: profile I, freezing rate from + 4°C to -40°C at 10°C min-1 and in profile II freezing rate from + 4 °C to -40°C at 15 °C min-1. The frozen samples in each experiment were plunged into the liquid nitrogen. |
Water bath at 25 °C for 30 seconds |
4.68 g L-1 NaCl, 2.98 g L-1 KCl, 0.11 g L-1 CaCl2 and 3.15 g L-1 Trizma-HCl, in distilled water (pH 9.0) (4) supplemented with 10% DMSO, Boron (0.1, 0.2, 0.3, and 0.4 mM) |
4.68 g L-1 NaCl, 2.98 g L-1 KCl, 0.11 g L-1 CaCl2, and 3.15 g L-1 Trizma-HCl in distilled water (pH 9.0) supplemented with 10% DMSO |
| Bucak8 |
Turkey |
Case-control |
Merino ram |
6 |
Equilibrated for 2 hours at 4⁰C, frozen in liquid nitrogen vapor for 15 minutes and stored at -196⁰C (liquid nitrogen)
for at least 1 month |
Thawed at 37 ⁰C for 30 seconds |
Tris-based freezing extender
first four groups: 5% glycerol + 0mM boron (G5B0.00), 5% glycerol + 0.25 mM boron (G5B0.25), 5% glycerol + 0.5 mM boron (G5B0.50), and 5% glycerol + 1mM boron (G5B1.00
The second four groups: supplementation of 60 mM trehalose plus 3% glycerol + 0 mM boron (G3B.00), 3% glycerol + 0.25 mM boron (G3B0.25), 3% glycerol + 0.5 mM boron (G3B0.50), and 3% glycerol + 1 mM boron (G3B1.00) |
Tris-based freezing extender |
| Karaşör17 |
Turkey |
Case-control |
Ankara buck |
5 |
Equilibrated at 5 ⁰C for 2–3 hours. After
frozen in liquid nitrogen vapors (-100 ⁰C) for 15 minutes and then plunged into liquid nitrogen for storage |
37 ⁰C for 30 seconds |
Six groups: Tris-based extender solution plus ROCK inhibitor (5 or 20 µM), antifreeze protein III (1 or 4 µg/mL), boron (0.25 or 1 mM) |
Tris-based extender solution consisting of 82.66 mM fructose, 96.32 mM citric acid,
297.58 mM Tris, 15% egg yolk, 1% penicillin-streptomycin-amphotericin B mixture, and
5% glycerol |
Results
Research Inclusion
A total of 1602 citations were identified through electronic, manual, and reference checks. After removing duplicates, 919 studies remained for screening. From this pool, 35 studies were selected based on the information provided in their titles and abstracts. However, after a thorough examination of the entire texts, 30 articles were not included in the final selection. The final analysis included five studies, combining both the current study and the critical assessment approach. The PRISMA flowchart provides a more detailed representation of the selection process (Figure 1).
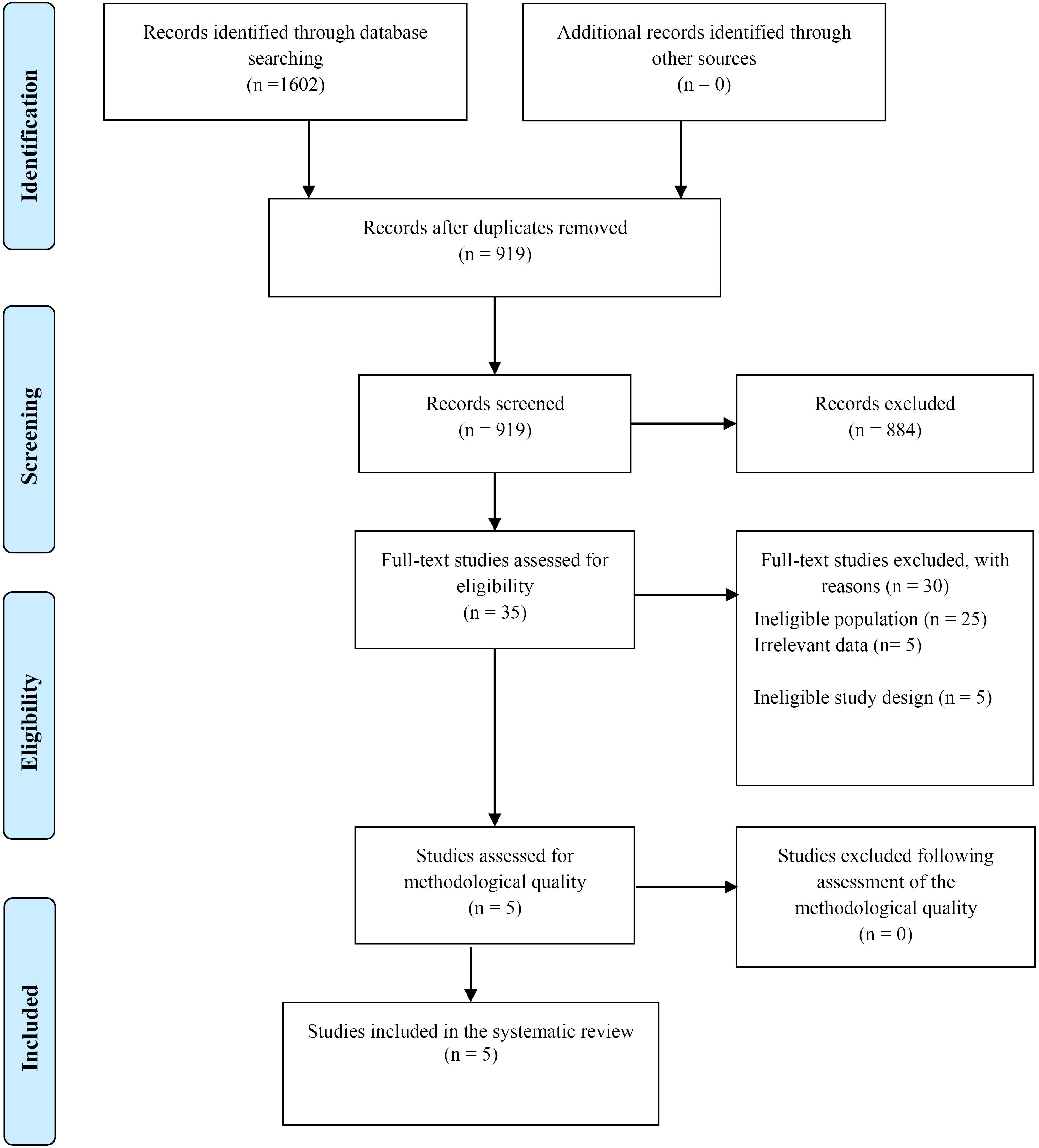
Figure 1.
PRISMA Flow Diagram. Note. PRISMA: Preferred reporting items for systematic reviews and meta-analyses
.
PRISMA Flow Diagram. Note. PRISMA: Preferred reporting items for systematic reviews and meta-analyses
Characteristics of the Included Studies
All five studies included in the analysis were conducted in Turkey and used a case-control design. The first study used a sample of 10 Angora goats. The sample sizes for the remaining four studies were as follows: 4 bulls, 12 brown trouts, 5 Merino rams, and 5 Ankara bucks. Table 1 offers the details of the included studies.
Tirpan and colleagues’32 study involved freezing the semen of Angora goats using four boron-added extenders (BAD) with varying ingredients (1a-d), four extenders with boron replaced glucose (GIB) in Tris extender (2a-d), and a control extender. For groups 1a–d, the osmotic pressure (mOsmol), pH, and sodium pentaborate (g) were as follows:
-
1a: 305, 7.28, 0.3
-
1b: 304, 7.21, 0.5
-
1c: 331, 7.08, 0.7
-
1d: 342, 7.05, 0.9.
The groups 2a-d had the following values:
-
2a: 338, 7.26, 0.2
-
2b: 335, 7.21, 0.4
-
2c: 349, 7.12, 0.6
-
2d: 327, 7.03, 0.8
The mOsmol, pH, and sodium pentaborate of the control extender were 333, 7.20, and 0, respectively. The glucose content in all extenders was 0.5 g, except for the GIB extenders, where the glucose content was zero. In addition, the quantities of Tris and citric acid in all extenders were 3.63 g and 1.82 g, respectively. The findings indicated that the extenders 1c and 1d yielded the highest values for total motility and progressive motility, respectively. The semen’s kinetic parameters were highest in the 2d extender, while the lowest values were observed in the control extender. The lowest ratio of nonviable spermatozoa was found in extenders 2-b and 1-c, whereas the highest ratio was noticed in extender 2-d. The ratios of abnormal spermatozoa were lower than those in the control group in all extenders, except for extender 2-b. Furthermore, all extenders exhibited a decreased proportion of abnormal spermatozoa with respect to the acrosome compared to the control group. In summary, the study indicates that the use of BAD and GIB extenders can enhance the post-thaw motility characteristics of spermatozoa in Angora goat semen.32
In a separate study conducted by Tirpan et al, the researchers assessed the impact of various boron doses in bull semen extenders on sperm parameters after thawing. The study involved five extender groups: one control group without boron and four groups with boron doses of 0.4, 0.5, 0.7, and 0.9 grams, respectively. The findings indicated that group 1, which had the lowest boron content, exhibited the greatest values for straight-line velocity (VSL), linearity (LIN), straightness (STR), and wobble (WOB), whereas the control group displayed the lowest values. Group 1 also indicated the highest levels of curvilinear velocity (VCL), average path velocity (VAP), amplitude of lateral head (ALH), and beating frequency (BCF). However, the differences between groups did not reach statistical significance. Furthermore, group 1 exhibited the highest values for total motile sperm count (TMOT) and progressively motile sperm count (PMOT), whereas the control group had the lowest values. The PMAI score was greater in group 1 compared to the control and other groups. Nonetheless, there were no significant variations in DFI values throughout the groups. In summary, their research indicated that incorporating a minimal quantity of boron into bull semen extenders could enhance post-thaw sperm characteristics, particularly with regard to motility.33
Bozkurt et al conducted a study to assess the impact of boron on various aspects of brown trout spermatozoa after cryopreservation using two distinct freezing profiles. The researchers examined post-thaw motility, motility duration, viability, fertility, and DNA integrity. Profile I involved a freezing rate ranging from + 4°C to -40°C at 10°C per minute, while Profile II had a freezing rate ranging from + 4°C to -40°C at 15°C per minute. After freezing, each experiment’s frozen samples were submerged in liquid nitrogen. Boron was individually introduced into the extender at doses of 0.1, 0.2, 0.3, and 0.4 mM. The findings demonstrated that the incorporation of boron into the extender improved post-thaw sperm characteristics in brown trout. More precisely, sperm treated with 0.4 mM boron exhibited the highest levels of post-thaw motility, the longest motility duration, and the highest fertilization rates. Moreover, the most optimal viability and eyeing rates were observed at a boron concentration of 0.1 mM. The study also revealed that freezing profile-I led to notably superior post-thaw sperm parameters compared to freezing profile-II. Moreover, the study found that sperm preserved without boron, at both freezing rates, exhibited more significant DNA damage compared to the boron-treated groups. The addition of 0.4 mM boron to the extender reduced comet percentage and tail lengths in the frozen sperm when using freezing profile-I, as compared to the other groups.18
The study conducted by Bucak et al examined the impact of boron supplementation on various parameters of Merino ram spermatozoa following cryopreservation. The findings demonstrated that the inclusion of 0.25 mM boron, combined with trehalose (G3B0.25), led to increased levels of subjective motility, mitochondrial activity, and sperm viability compared to the other groups. Furthermore, the presence of boron at this specific concentration, with and without trehalose (G3B0.25 and G5B0.25), resulted in better maintenance of acrosome integrity compared to the other experimental groups. The study also found that increasing the boron dosages with 3% glycerol tended to reduce DNA damage, as measured by the TUNEL assay, though this effect was not statistically significant. Nevertheless, the group with 1.00 mM boron (G3B1.00) exhibited the highest DNA integrity compared to the other experimental groups, as determined by TUNEL analysis.12 No substantial disparities in DNA damage were observed across the freeze-thawing process, as indicated by the COMET assay. In addition, the study evaluated the impact of boron on the expression of NQO1, GCLC, and GSTP1 genes. The mRNA levels of NQO1 exhibited a significant reduction in groups receiving boron supplementation compared to the group. Furthermore, the inclusion of 1 mM boron, with or without trehalose, in the cryopreservation extender resulted in a marked reduction in the expression of the GCLC gene compared to the group without boron supplementation. The expression of the GSTP1 gene was also markedly reduced in the G3B1.00 group compared to the control group without boron supplementation.12
According to Karaşör et al, the addition of 0.25 and 1 mM boron demonstrated better protection than the control group. Following the freeze-thawing process, the sperm plasma membrane, acrosome integrity, and mitochondrial membrane activity were assessed. Although none of the boron concentrations added to the extender showed statistically significant efficacy, when sperm DNA damage was assessed following the freeze-thaw process, all boron concentrations offered greater cryoprotective benefits than the control group.17 A summary of the key findings from the included studies is provided in Table 2.
Table 2.
Summary of the Results
|
No.
|
Author (year)
|
Outcome Measure
|
Result
|
| 1 |
Tirpan32 |
Motility (%) |
1a: 41.27 ± 1.65, 1b: 56.97 ± 1.55, 1c: 63.34 ± 2.32*, 1d: 58.43 ± 0.84
2a: 41.73 ± 1.11, 2b: 59.56 ± 2.20, 2c: 41.44 ± 6.26, 2d: 32.29 ± 4.18*
Control: 47.89 ± 2.17 |
|
|
|
Progressive Motility (%) |
1a: 19.50 ± 2.07, 1b: 25.26 ± 1.77, 1c: 29.46 ± 1.58*, 1d: 38.27 ± 0.74*
2a: 21.77 ± 1.68, 2b: 30.99 ± 2.11*, 2c: 24.46 ± 4.26, 2d: 18.39 ± 2.63
Control: 17.86 ± 0.77 |
|
|
|
VCL (μm/s) |
1a: 125.91 ± 7.26*, 1b: 129.70 ± 3.57*, 1c: 124.71 ± 5.45*, 1d: 122.47 ± 5.18*
2a: 125.71 ± 6.55*, 2b: 122.26 ± 3.55*, 2c: 137.61 ± 1.87*, 2d: 146.40 ± 3.37*
Control: 91.79 ± 3.01 |
|
|
|
VSL (μm/s) |
1a: 79.93 ± 5.27*, 1b: 81.99 ± 3.09*, 1c: 83.46 ± 4.99*, 1d: 80.36 ± 4.51*
2a: 76.80 ± 6.54*, 2b: 70.37 ± 4.64*, 2c: 78.80 ± 2.05*, 2d: 100.29 ± 1.50*
Control: 54.04 ± 1.77 |
|
|
|
VAP (μm/s) |
1a: 98.76 ± 6.17*, 1b: 102.90 ± 3.43*, 1c: 99.21 ± 5.60*, 1d: 95.23 ± 4.91*
2a: 97.33 ± 6.33*, 2b: 90.84 ± 5.20*, 2c: 100.94 ± 2.07*, 2d: 117.93 ± 2.07*
Control: 68.66 ± 2.25 |
|
|
|
LIN (%) |
1a: 63.59 ± 2.07, 1b: 63.16 ± 1.10, 1c: 66.71 ± 1.55*, 1d: 65.60 ± 2.15*
2a: 60.81 ± 3.24, 2b: 57.21 ± 2.31, 2c: 57.21 ± 0.89, 2d: 68.60 ± 0.87*
Control: 58.93 ± 1.05 |
|
|
|
STR (%) |
1a:81.04 ± 1.71, 1b: 79.66 ± 0.75, 1c: 84.04 ± 0.78*, 1d: 84.30 ± 1.10*
2a: 78.40 ± 2.01, 2b: 77.23 ± 0.81, 2c: 78.03 ± 0.84, 2d: 85.06 ± 0.35*
Control: 78.73 ± 0.60 |
|
|
|
WOB (%) |
1a: 78.39 ± 1.29, 1b: 79.30 ± 0.82, 1c: 79.31 ± 1.26, 1d: 77.69 ± 1.60
2a: 77.24 ± 2.12, 2b: 73.96 ± 2.30, 2c: 73.31 ± 0.87, 2d: 80.63 ± 0.72*
Control: 74.83 ± 0.84 |
|
|
|
Dead spermatozoa(%) |
1a: 51.21 ± 2.16, 1b: 40.70 ± 2.15, 1c: 36.17 ± 3.66*, 1d: 40.61 ± 1.52
2a: 52.76 ± 2.03, 2b: 36.04 ± 2.58*, 2c: 55.23 ± 4.97, 2d: 62.03 ± 3.61*
Control: 49.23 ± 1.63 |
|
|
|
Spermatozoon abnormality (%) |
1a: 27.14 ± 1.18*, 1b: 29.71 ± 1.21, 1c: 24.86 ± 1.72*, 1d: 28.71 ± 1.81
2a: 24.57 ± 0.90, 2b: 21.29 ± 0.97*, 2c: 28.57 ± 2.53, 2d: 37.14 ± 1.20
Control: 33.43 ± 1.17 |
|
|
|
Acrosomal damage (%) |
1a: 6.57 ± 1.29*, 1b: 10.43 ± 2.22, 1c: 7.43 ± 1.78*, 1d: 6.71 ± 2.00*
2a: 13.29 ± 1.89, 2b: 7.86 ± 1.94, 2c: 9.29 ± 1.04, 2d: 12.00 ± 0.44
Control: 15.14 ± 1.87 |
|
|
|
Conclusion |
Sodium pentaborate used in the study may be an alternative substance for the extenders, positively influencing some parameters without negative effects when freezing-thawing semen parameters are considered. |
| 2 |
Tirpan33 |
TMOT |
G1: 66.04 ± 5.95, G2: 60.47 ± 5.45, G3: 62.11 ± 4.90, G4: 61.99 ± 3.26
Control: 49.79 ± 6.71 |
|
|
|
PMOT |
G1: 50.80 ± 4.71, G2: 44.77 ± 4.10, G3: 46.92 ± 3.90, G4: 47.02 ± 2.79
Control: 34.38 ± 4.56 |
|
|
|
VCL (μm/s) |
G1: 65.81 ± 2.27, G2: 64.55 ± 3.40, G3: 64.05 ± 2.15, G4: 62.08 ± 1.59
Control: 65.46 ± 3.33 |
|
|
|
VSL (μm/s) |
G1: 28.15 ± 1.08*, G2: 23.90 ± 1.26, G3: 24.26 ± 0.96, G4: 24.50 ± 1.05
Control: 22.06 ± 1.17 |
|
|
|
VAP (μm/s) |
G1: 39.31 ± 1.49, G2: 35.70 ± 1.88, G3: 35.49 ± 1.20, G4: 34.85 ± 1.00
Control: 35.68 ± 1.91 |
|
|
|
LIN (%) |
G1: 42.98 ± 1.31*, G2: 37.14 ± 1.31, G3: 37.96 ± 1.15, G4: 39.44 ± 1.20*
Control: 33.80 ± 1.16 |
|
|
|
STR (%) |
G1: 71.69 ± 1.16*, G2: 66.99 ± 1.48, G3: 68.33 ± 1.25*, G4: 70.13 ± 1.31*
Control: 61.94 ± 1.05 |
|
|
|
WOB (%) |
G1: 59.71 ± 1.06*, G2: 55.35 ± 0.87, G3: 55.45 ± 0.73, G4: 56.15 ± 0.71
Control: 54.48 ± 1.09 |
|
|
|
ALH (μm) |
G1: 2.86 ± 0.07, G2: 3.00 ± 0.12, G3: 3.00 ± 0.09, G4: 2.93 ± 0.08
Control: 3.04 ± 0.11 |
|
|
|
BCF (Hz) |
G1: 9.73 ± 0.17, G2: 9.60 ± 0.28, G3: 9.55 ± 0.24, G4: 9.60 ± 0.30
Control: 8.71 ± 0.27 |
|
|
|
Plasma membrane and acrosome integrity (%) |
G1: 60.84 ± 2.90, G2: 57.89 ± 3.39, G3: 58.67 ± 5.18, G4: 59.45 ± 2.58
Control: 48.27 ± 3.83 |
|
|
|
DNA fragmentation index
(%) |
G1: 3.20 ± 0.38, G2: 3.48 ± 0.43, G3: 3.49 ± 0.46, G4: 3.57 ± 0.47
Control: 3.18 ± 0.47 |
|
|
|
Conclusion |
Boron (sodium pentaborate) has positive effects on post-thaw movement and structural traits. In addition, boron has no detrimental effect on DNA fragmentation index in semen freezing. |
| 3 |
Bozkurt18 |
Comet length (μm) |
Freezing Rate I: 0.1B: 63.18 ± 2.32*, 0.2B: 61.78 ± 2.48*, 0.3B: 61.70 ± 3.34*, 0.4B: 46.57 ± 2.25*
Control: 68.4 ± 3.20
Freezing Rate II: 0.1B:74.32 ± 2.69*, 0.2B: 71.42 ± 3.39*, 0.3B: 70.10 ± 1.89*, 0.4B:67.24 ± 1.48*
Control: 88.70 ± 4.68 |
|
|
|
Tail Length (μm) |
Freezing Rate I: 0.1B: 14.92 ± 1.83*, 0.2B: 13.84 ± 1.81*, 0.3B: 10.16 ± 1.79*, 0.4B: 4.32 ± 0.91*
Control: 22.20 ± 1.78
Freezing Rate II: 0.1B: 7.04 ± 1.88*, 0.2B: 6.44 ± 1.46*, 0.3B: 5.94 ± 1.53*, 0.4B: 2.14 ± 0.52*
Control: 18.46 ± 1.54 |
|
|
|
Tail DNA (%) |
Freezing Rate I: 0.1B: 1.64 ± 0.48, 0.2B: 0.48 ± 0.27, 0.3B: 0.36 ± 0.27, 0.4B: 0.35 ± 0.14
Control: 2.41 ± 0.64
Freezing Rate II: 0.1B: 29.06 ± 3.50, 0.2B: 23.34 ± 2.69*, 0.3B: 10.42 ± 1.56*, 0.4B: 7.82 ± 0.27*
Control: 29.70 ± 2.38 |
|
|
|
Tail moment (unit) |
Freezing Rate I: 0.1B: 0.45 ± 0.14, 0.2B: 0.09 ± 0.06, 0.3B: 0.09 ± 0.04, 0.4B: 0.08 ± 0.07
Control: 0.65 ± 0.22
Freezing Rate II: 0.1B: 1.63 ± 0.48, 0.2B: 1.27 ± 0.48, 0.3B: 0.86 ± 0.29, 0.4B: 0.80 ± 0.25
Control: 2.20 ± 0.55 |
|
|
|
Olive Tail moment (% Tail DNA/100) |
Freezing Rate I: 0.1B: 0.47 ± 0.14, 0.2B: 0.14 ± 0.06, 0.3B: 0.12 ± 0.08, 0.4B: 0.10 ± 0.07
Control: 0.65 ± 0.17
Freezing Rate II: 0.1B: 4.61 ± 0.67, 0.2B: 2.91 ± 0.42*, 0.3B: 1.48 ± 0.36*, 0.4B: 1.34 ± 0.28*
Control: 4.73 ± 0.64 |
|
|
|
Overall result and conclusion |
Freezing Rate-I provided significantly higher fertilization and eyeing rates compared to freezing rate-II (P < 0.05). Higher post-thaw motility (62.8 ± 1.4%) and fertilization (75.2 ± 0.9%) rates were obtained with the 0.4 mM boron concentration at freezing rate-I. Supplementation of the extender with boron increased fertilization and eyeing rates and also decreased DNA damage at both freezing rates. |
| 4 |
Bucak8 |
Overall result and conclusion |
Supplementation with 0.25 mM boron, with and without trehalose (G3B0.25 and G5B0.25), showed higher acrosome integrity, compared to G5B0.00, G5B1.00, G3B0.50, and G3B1.00. In TUNEL analysis, G3B1.00 exhibited the highest DNA integrity among the experimental groups, which was statistically significant only with G5B0.50 (P < 0.05). The mRNA levels of NQO1 were significantly decreased in G5B1.00, G3B0.50, and G3B1.00 compared to G5B0.00. Additionally, supplementation with 1 mM boron, with and without trehalose, significantly reduced the expression of GCLC compared to G5B0.00. The GSTP1 gene level was significantly lower (approximately threefold) in G3B1.00 compared to G5B0.00 (P < 0.05). These findings suggest that higher boron concentrations in the extender may have important adverse effects on sperm parameters and antioxidant gene expression after thawing. The use of sufficient boron can decrease cryo-damages in the cryopreservation of mammalian spermatozoa as well in tissue engineering. |
| 5 |
Karasör17 |
Overall result and conclusion |
Differences were significant for the 0.25 and 1 mM doses of boron (76.36% and 72.08% motility, respectively), compared to the control group (66.15% motility). DNA damage was improved by boron at all doses (0.25 mM: 1.83% and 1 mM: 1.18%) compared to the control group (3.37%) (P < 0.01), following the thawing process. The present study concluded that some additives added to the extender, including boron, significantly improved buck spermatozoa motility and reduced DNA damage after thawing. |
Note. VCL: Curvilinear velocity; VSL: Straight-line velocity; VAP: Average path velocity; LIN: Linearity; STR: Straightness; WOB: Wobble; TMOT: Total motile sperm count; PMOT: Progressively motile sperm count; ALH: Amplitude of lateral head displacement; BCF: Beating frequency.
*P < 0.05 compared to the control.
Risk of Bias in the Included Studies
Using the standardized ARRIVE Essential 10: Compliance Questionnaire appraisal tools, all the studies achieved an acceptable score, and none were excluded (Table 3).
Table 3.
Critical Appraisal Results of Eligible Studies
|
Study
|
Q1A
|
Q1B
|
Q2A
|
Q2B
|
Q3A
|
Q3B
|
Q4
|
Q5
|
Q6
|
Q7A
|
Q7B
|
Q8A
|
Q8B
|
Q8C
|
Q9A
|
Q9B
|
Q10A
|
Q10B
|
| Karasör 17 |
Y |
Y |
Y |
N |
Y |
Y |
Y |
N |
Y |
Y |
NA |
Y |
Y |
Y |
Y |
N |
Y |
NA |
| Bucak 8 |
Y |
Y |
Y |
N |
Y |
Y |
Y |
N |
Y |
Y |
NA |
Y |
Y |
Y |
Y |
N |
Y |
NA |
| Tirpan33 |
Y |
Y |
Y |
N |
Y |
Y |
Y |
N |
Y |
Y |
NA |
Y |
Y |
Y |
Y |
N |
Y |
NA |
| Tirpan32 |
Y |
Y |
Y |
N |
Y |
Y |
Y |
N |
Y |
Y |
NA |
Y |
Y |
Y |
Y |
N |
Y |
NA |
| Bozkurt18 |
Y |
Y |
Y |
N |
N |
Y |
Y |
N |
Y |
Y |
NA |
Y |
Y |
Y |
Y |
N |
Y |
NA |
Note. Y: Yes; N: No; U: Unclear; NA: Not applicable. The ARRIVE Essential 10: Compliance Questionnaire includes the following criteria: Q1A: Are all experimental and control groups clearly identified?; Q1B: Is the experimental unit (e.g. an animal, litter, or cage of animals) clearly identified?; Q2A: Is the exact number of experimental units in each group at the start of the study provided (e.g. in the format ‘n = ’)?; Q2B: Is the sample size method explained?; Q3A: Are the criteria for including/excluding animals, experimental units, or data points provided?; Q3B: Are exclusions of animals, experimental units, or data points reported? Or is there a statement indicating that there were no exclusions?; Q4: Is the allocation method of experimental units to control and treatment groups described?; Q5: Is it clear whether researchers were aware of, or blinded to, group allocation during the experiment or data analysis?; Q6: Are details provided for the measured parameters?; Q7A: Is the statistical approach detailed?; Q7B: Are methods to assess statistical assumptions described? Q8A: Are all species of animal used specified?; Q8B: Is the sex of the animals specified?; Q8C: Is the age, weight, or developmental stage of the animals specified?; Q9A = Are both the timing and frequency with which procedures took place specified?; Q9B: Are acclimatization details provided?; Q10A: Are descriptive statistics for each experimental group with variability provided (e.g. mean and SD, or median and range)?; Q10B = Are an effect size and confidence interval provided?
Discussion
The studies conducted in Turkey highlight the potential benefits of incorporating boron into semen extenders for various species, including Angora goats, bulls, brown trouts, Merino rams, and Ankara bucks. The results indicated that boron supplementation improved post-thaw sperm quality, particularly in terms of motility, viability, acrosome integrity, and DNA damage reduction. However, different concentrations of boron indicated varying effects on sperm parameters. Specifically, 0.4 mM boron yielded the best results for brown trout sperm, while 0.25 mM combined with trehalose proved optimal for Merino ram sperm.
Semen preservation through freezing and insemination at the optimal timing is critical for breeding and reproduction in specific animal species. This approach can also play a key role in managing male fertility. Boron, a naturally occurring element, has been found to enhance the quality of cryopreserved spermatozoa. The systematic review comprising five studies demonstrated favorable results, with improved quality parameters linked to boron as supplementation in semen extenders during cryopreservation.
Cryopreservation of sperm involves exposure to various stresses such as chemical toxicity, oxidative stress, and ice formation.1 Crystallization in the extracellular environment exerts pressure on both the cell and organelle membranes. This occurs when the ice crystals pass through the aquaporin pores in the membrane and enter the cell, resulting in membrane disruption due to osmotic pressure.2-4 Additionally, spermatozoa’s plasma, outer acrosomal, and mitochondrial membranes are susceptible to freeze-thaw. These membranes, composed of two layers of phospholipid intercalated with proteins, glycoproteins, and glycolipids, form a fluid mosaic membrane structure. Membrane cooling causes an irreversible liquid-to-gel phase shift, which alters intramembrane enzyme kinetics, lowering post-thawed sperm parameters.10,12,16
Antifreeze protein III protects against cold shock by inhibiting crystal formation, attaching to crystal structures, and decreasing freezing temperatures, thus preventing the formation of large crystal particles during freeze-thawing.17,19,22,23 It also protects membranes by blocking ion channels, preventing ions from leaking into the environment.25,27
The level of motility in sperm is a key indicator of its quality. Previous studies have demonstrated that cryopreserved spermatozoa exhibit poorer motility compared to fresh sperm.30-34 Motility is regulated in the mid-segment of the sperm, specifically in the regions of the flagellar and axoneme. The motility of spermatozoa is typically determined by energy continuity, structural integrity, and regulation.35 Across these studies, boron-added extenders have been found to enhance the post-thaw and progressive motility of spermatozoa, which is one of the key findings of these studies. This observation suggests that boron plays a significant role in preserving the structural integrity and functioning capacity of sperm cells throughout the freeze-thaw cycle. Additionally, studies have shown that boron supplementation can reduce the ratios of nonviable and abnormal spermatozoa, further highlighting its protective effect during cryopreservation. Karaşör et al17 reported that antifreeze protein III significantly preserves post-thaw motility, supporting the idea that boron-added extenders may influence this pathway and result in higher post-thaw motility compared to the control groups.
Interestingly, the studies also highlighted the significance of boron concentration. For instance, Bozkurt et al18 discovered that brown trout spermatozoa showed the highest motility and fertilization rates after being thawed when exposed to a boron concentration of 0.4 mM. In contrast, Bucak et al12 found that a boron concentration of 0.25 mM yielded the highest percentages for most post-thaw parameters in Merino ram sperm. These findings emphasize the importance of precisely optimizing boron concentrations in semen extenders for optimal outcomes. Furthermore, they highlight that the ideal concentration of boron may vary across species and should be optimized.
Mitochondrial oxidative phosphorylation generates the energy needed for sperm motility. Free radicals generated during the freezing-thawing process decrease ATP, damaging axonemal tissue and causing loss of motility.34 Bucak et al8 found that mitochondrial function may be preserved by low dosages of boron in a low-glycerol environment. Karasör et al also found that boron supplementation preserved mitochondrial activity, though the effect was not significant.17 Sperm DNA damage and decreased fertility and pregnancy rates are biomarkers for male infertility.35-37 Previous studies have shown that DNA damage such as condensation, fragmentation, and strand breaks increases after freezing spermatozoa, likely due to the volume of seminal fluid and the absence of natural antioxidants in seminal fluid.38 In another study, Bucak et al12 and Bozkurt et al18 demonstrated that increased boron content reduces DNA damage. Yeni et al froze the Ramlıç ram spermatozoa in different boron diluent concentrations (1, 2, and 4 mM) added to a Tris-based extender with 5% glycerol. They also froze sperm with 1 mM boron diluent containing 3% glycerol and 60 mM trehalose. Their findings suggested that higher concentrations of boron and trehalose may have a synergistic impact on sperm DNA integrity.39
Antioxidant genes play a pivotal role in defending cells against oxidative stress. The NQO1 gene is crucial for combating oxidative damage through its involvement in the NADPH dehydrogenase family. Its product facilitates the reduction of quinones to hydroquinones, thereby preventing cellular damage.40-43 Based on the cellular response to oxidative stress, nuclear Nrf2 pathway upregulation of the GCLC gene increases testicular antioxidant capacity. Previous studies showed that GCLC overexpression in frozen-thawed spermatozoa protects them against chilling and oxidative stress. The GCLC gene, part of the glutathione metabolic pathway, may also increase in bull spermatozoa after freeze-thaw treatment.44-46 This review highlights the impact of boron on gene expression in spermatozoa. For instance, boron supplementation reduced the expression of genes such as NQO1, GCLC, and GSTP1, which are associated with oxidative stress and sperm function.4 This observation provides a molecular basis for the protective role of boron and its potential to enhance spermatozoa resilience to cryopreservation-induced stress.
Conclusion
In sum, this systematic review indicates that adding boron to semen extenders can significantly enhance the quality of cryopreserved spermatozoa. Nevertheless, further investigation is required to determine the ideal concentration of boron for yielding the maximum benefits. These findings contribute to improving cryopreservation techniques, which are crucial for fertility preservation in both humans and animals.
Authors’ Contribution
Conceptualization: Mustafa Numan Bucak.
Data curation: Anis Sani, Amin Sani, Keysan Pourmoghtader.
Formal analysis: Hanieh Salehi-Pourmehr, Sakineh Hajebrahimi.
Investigation: Mustafa Numan Bucak, Hanieh Salehi-Pourmehr.
Methodology: Keysan Pourmoghtader, Mohammad Hamidi Madani, Reza Aletaha.
Project administration: Mustafa Numan Bucak.
Resources: Anis Sani, Amin Sani.
Software: Hanieh Salehi-Pourmehr.
Supervision: Mustafa Numan Bucak, Hanieh Salehi-Pourmehr, Sakineh Hajebrahimi.
Validation: Mustafa Numan Bucak, Hanieh Salehi-Pourmehr, Sakineh Hajebrahimi.
Visualization: Mustafa Numan Bucak, Hanieh Salehi-Pourmehr, Sakineh Hajebrahimi.
Writing–original draft: Anis Sani, Amin Sani, Keysan Pourmoghtader, Mohammad Hamidi Madani, Reza Aletaha.
Writing–review & editing: Mustafa Numan Bucak, Hanieh Salehi-Pourmehr, Sakineh Hajebrahimi.
Ethical Approval
This study is approved by regional committee of Tabriz University of Medical Sciences (IR.TBZMED.REC.1403.050).
References
- Khan IM, Cao Z, Liu H, Khan A, Rahman SU, Khan MZ. Impact of cryopreservation on spermatozoa freeze-thawed traits and relevance OMICS to assess sperm cryo-tolerance in farm animals. Front Vet Sci 2021; 8:609180. doi: 10.3389/fvets.2021.609180 [Crossref] [ Google Scholar]
- World Health Organization (WHO). WHO Laboratory Manual for the Examination and Processing of Human Semen. WHO; 2010.
- Oktay K, Harvey BE, Partridge AH, Quinn GP, Reinecke J, Taylor HS. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol 2018; 36(19):1994-2001. doi: 10.1200/jco.2018.78.1914 [Crossref] [ Google Scholar]
- Watson PF, Morris GJ. Cold shock injury in animal cells. Symp Soc Exp Biol 1987; 41:311-40. [ Google Scholar]
- AbdelHafez F, Bedaiwy M, El-Nashar SA, Sabanegh E, Desai N. Techniques for cryopreservation of individual or small numbers of human spermatozoa: a systematic review. Hum Reprod Update 2009; 15(2):153-64. doi: 10.1093/humupd/dmn061 [Crossref] [ Google Scholar]
- Prieto-Martínez N, Vilagran I, Morató R, Rodríguez-Gil JE, Yeste M, Bonet S. Aquaporins 7 and 11 in boar spermatozoa: detection, localisation and relationship with sperm quality. Reprod Fertil Dev 2016; 28(6):663-72. doi: 10.1071/rd14237 [Crossref] [ Google Scholar]
- Medeiros CM, Forell F, Oliveira AT, Rodrigues JL. Current status of sperm cryopreservation: why isn’t it better?. Theriogenology 2002; 57(1):327-44. doi: 10.1016/s0093-691x(01)00674-4 [Crossref] [ Google Scholar]
- Bucak MN, Keskin N, Bodu M, Bülbül B, Kırbaş M, Öztürk AE. Combination of trehalose and low boron in presence of decreased glycerol improves post-thawed ram sperm parameters: a model study in boron research. Andrology 2022; 10(3):585-94. doi: 10.1111/andr.13130 [Crossref] [ Google Scholar]
- Hezavehei M, Sharafi M, Mohseni Kouchesfahani H, Henkel R, Agarwal A, Esmaeili V. Sperm cryopreservation: a review on current molecular cryobiology and advanced approaches. Reprod Biomed Online 2018; 37(3):327-39. doi: 10.1016/j.rbmo.2018.05.012 [Crossref] [ Google Scholar]
- Öztürk AE, Bucak MN, Bodu M, Başpınar N, Çelik İ, Shu Z, et al. Cryobiology and cryopreservation of sperm. In: Quain M, ed. Cryopreservation-Current Advances and Evaluations. IntechOpen; 2019. p. 75116.
- Valipour J, Shabani Nashtaei M, Khosravizadeh Z, Mahdavinezhad F, Nekoonam S, Esfandyari S. Effect of sulforaphane on apoptosis, reactive oxygen species and lipids peroxidation of human sperm during cryopreservation. Cryobiology 2021; 99:122-30. doi: 10.1016/j.cryobiol.2020.11.012 [Crossref] [ Google Scholar]
- Bucak MN, Tekin N. Kryoprotektanlar ve gamet hücrelerinin dondurulmasında kryoprotektif etki. Ankara Univ Vet Fak Derg 2007; 54(1):67-72. doi: 10.1501/Vetfak_0000000255 [Crossref] [ Google Scholar]
- Tuncer PB, Bucak MN, Sariözkan S, Sakin F, Yeni D, Ciğerci IH. The effect of raffinose and methionine on frozen/thawed Angora buck (Capra hircusancryrensis) semen quality, lipid peroxidation and antioxidant enzyme activities. Cryobiology 2010; 61(1):89-93. doi: 10.1016/j.cryobiol.2010.05.005 [Crossref] [ Google Scholar]
- Keskin N, Erdogan C, Bucak MN, Öztürk AE, Bodu M, Ili P. Cryopreservation effects on ram sperm ultrastructure. Biopreserv Biobank 2020; 18(5):441-8. doi: 10.1089/bio.2020.0056 [Crossref] [ Google Scholar]
- Bucak MN, Tuncer PB, Sariözkan S, Ulutaş PA, Coyan K, Başpinar N. Effects of hypotaurine, cysteamine and aminoacids solution on post-thaw microscopic and oxidative stress parameters of Angora goat semen. Res Vet Sci 2009; 87(3):468-72. doi: 10.1016/j.rvsc.2009.04.014 [Crossref] [ Google Scholar]
- Bharudin I, Abu Bakar MF, Hashim NHF, Mat Isa MN, Alias H, Firdaus-Raih M. Unravelling the adaptation strategies employed by Glaciozyma antarctica PI12 on Antarctic sea ice. Mar Environ Res 2018; 137:169-76. doi: 10.1016/j.marenvres.2018.03.007 [Crossref] [ Google Scholar]
- Karaşör ÖF, Bucak MN, Cenariu M, Bodu M, Taşpınar M, Taşpınar F. The effects of different doses of ROCK inhibitor, antifreeze protein III, and boron added to semen extender on semen freezeability of Ankara bucks. Molecules 2022; 27(22):8070. doi: 10.3390/molecules27228070 [Crossref] [ Google Scholar]
- Bozkurt Y, Yavas I, Gul A, Bucak MN, Yeni D, Avdatek F. Effect of extender supplemented with boron on post-thaw motility, viability, DNA damage and fertilization ability of cryopreserved brown trout (Salmo trutta macrostigma) spermatozoa. Cryo Letters 2019; 40(5):275-83. [ Google Scholar]
- Koushafar H, Pham L, Lee C, Rubinsky B. Chemical adjuvant cryosurgery with antifreeze proteins. J Surg Oncol 1997; 66(2):114-21. doi: 10.1002/(sici)1096-9098(199710)66:2<114::aid-jso8>3.0.co;2-g [Crossref] [ Google Scholar]
- Korkmaz M, Sayli U, Sayli BS, Bakirdere S, Titretir S, Yavuz Ataman O. Estimation of human daily boron exposure in a boron-rich area. Br J Nutr 2007; 98(3):571-5. doi: 10.1017/s000711450770911x [Crossref] [ Google Scholar]
- Korkmaz M, Yenigün M, Bakırdere S, Yavuz Ataman O, Keskin S, Müezzinoğlu T. Effects of chronic boron exposure on semen profile. Biol Trace Elem Res 2011; 143(2):738-50. doi: 10.1007/s12011-010-8928-2 [Crossref] [ Google Scholar]
- Ustun NS, Turhan S. Antifreeze proteins: characteristics, function, mechanism of action, sources and application to foods. J Food Process Preserv 2015; 39(6):3189-97. doi: 10.1111/jfpp.12476 [Crossref] [ Google Scholar]
- Crevel RW, Fedyk JK, Spurgeon MJ. Antifreeze proteins: characteristics, occurrence and human exposure. Food Chem Toxicol 2002; 40(7):899-903. doi: 10.1016/s0278-6915(02)00042-x [Crossref] [ Google Scholar]
- World Health Organization (WHO). Trace Elements in Human Nutrition and Health. WHO; 1996.
- Rubinsky B, Arav A, Fletcher GL. Hypothermic protection--a fundamental property of “antifreeze” proteins. Biochem Biophys Res Commun 1991; 180(2):566-71. doi: 10.1016/s0006-291x(05)81102-7 [Crossref] [ Google Scholar]
- Elkomy AE, Abd El-hady AM, Elghalid OA. Dietary boron supplementation and its impact on semen characteristics and physiological status of adult male rabbits. Asian J Poultry Sci 2015; 9(2):85-96. doi: 10.3923/ajpsaj.2015.85.96 [Crossref] [ Google Scholar]
- Rubinsky B, Arav A, Mattioli M, Devries AL. The effect of antifreeze glycopeptides on membrane potential changes at hypothermic temperatures. Biochem Biophys Res Commun 1990; 173(3):1369-74. doi: 10.1016/s0006-291x(05)80939-8 [Crossref] [ Google Scholar]
- Weir RJ Jr, Fisher RS. Toxicologic studies on borax and boric acid. Toxicol Appl Pharmacol 1972; 23(3):351-64. doi: 10.1016/0041-008x(72)90037-3 [Crossref] [ Google Scholar]
- Price CJ, Strong PL, Marr MC, Myers CB, Murray FJ. Developmental toxicity NOAEL and postnatal recovery in rats fed boric acid during gestation. Fundam Appl Toxicol 1996; 32(2):179-93. doi: 10.1006/faat.1996.0121 [Crossref] [ Google Scholar]
- Cabrita E, Robles V, Alvarez R, Herráez MP. Cryopreservation of rainbow trout sperm in large volume straws: application to large scale fertilization. Aquaculture 2001; 201(3-4):301-14. doi: 10.1016/S0044-8486(01)00636-6 [Crossref] [ Google Scholar]
- Nynca J, Dietrich GJ, Dobosz S, Grudniewska J, Ciereszko A. Effect of cryopreservation on sperm motility parameters and fertilizing ability of brown trout semen. Aquaculture 2014; 433:62-5. doi: 10.1016/j.aquaculture.2014.05.037 [Crossref] [ Google Scholar]
- Tirpan Mb, Teki̇n Teki̇n. Effects of boron (sodium pentaborate), added instead of Tris components, on freezing and post-thaw quality of Angora buck semen. Ankara Universitesi Veteriner Fakultesi DergisI 2015; 62(4):295-302. [ Google Scholar]
- Tırpan MB, Gürler H, Olğaç KT, Daşkın A. Effects of boron added bull semen extender on post-thaw spermatological parameters Ankara Üniversitesi Veteriner Fakültesi Dergisi. 2018 J un 1; 65(2):123-8. [ Google Scholar]
- Dziewulska K, Domagała J. Effect of pH and cation concentrations on spermatozoan motility of sea trout (Salmo trutta m trutta L). Theriogenology 2013; 79(1):48-58. doi: 10.1016/j.theriogenology.2012.09.008 [Crossref] [ Google Scholar]
- Suarez SS, Marquez B, Harris TP, Schimenti JC. Different regulatory systems operate in the midpiece and principal piece of the mammalian sperm flagellum. Soc Reprod Fertil Suppl 2007; 65:331-4. [ Google Scholar]
- Garner DL, Hafez ES. Spermatozoa and seminal plasma. In: Hafez B, Hafez ES, eds. Reproduction in Farm Animals. John Wiley & Sons; 2000. p. 96-109.
- Lewis SE. The place of sperm DNA fragmentation testing in current day fertility management. Middle East Fertil Soc J 2013; 18(2):78-82. doi: 10.1016/j.mefs.2013.01.010 [Crossref] [ Google Scholar]
- Kopeika J, Thornhill A, Khalaf Y. The effect of cryopreservation on the genome of gametes and embryos: principles of cryobiology and critical appraisal of the evidence. Hum Reprod Update 2015; 21(2):209-27. doi: 10.1093/humupd/dmu063 [Crossref] [ Google Scholar]
- Yeni D, Avdatek F, Gündoğan M. The effect of boron addition on spermatological parameters, oxidative stress and DNA damage after frozen-thawed process in Ramlic ram semen. Firat Universitesi Saglik Bilimleri Veteriner Dergisi 2018; 32(1):53-7. [ Google Scholar]
- Krajka-Kuźniak V, Paluszczak J, Szaefer H, Baer-Dubowska W. The activation of the Nrf2/ARE pathway in HepG2 hepatoma cells by phytochemicals and subsequent modulation of phase II and antioxidant enzyme expression. J Physiol Biochem 2015; 71(2):227-38. doi: 10.1007/s13105-015-0401-4 [Crossref] [ Google Scholar]
- Ross D, Kepa JK, Winski SL, Beall HD, Anwar A, Siegel D. NAD(P)H:quinone oxidoreductase 1 (NQO1): chemoprotection, bioactivation, gene regulation and genetic polymorphisms. Chem Biol Interact 2000; 129(1-2):77-97. doi: 10.1016/s0009-2797(00)00199-x [Crossref] [ Google Scholar]
- Dinkova-Kostova AT, Talalay P. NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch Biochem Biophys 2010; 501(1):116-23. doi: 10.1016/j.abb.2010.03.019 [Crossref] [ Google Scholar]
- Bahmani M, Mehrtabar S, Jafarizadeh A, Zoghi S, Sadeghpour Heravi F, Abbasi A. The gut microbiota and major depressive disorder: current understanding and novel therapeutic strategies. Curr Pharm Biotechnol 2024; 25(16):2089-107. doi: 10.2174/0113892010281892240116081031 [Crossref] [ Google Scholar]
- Nakamura BN, Lawson G, Chan JY, Banuelos J, Cortés MM, Hoang YD. Knockout of the transcription factor Nrf2 disrupts spermatogenesis in an age-dependent manner. Free Radic Biol Med 2010; 49(9):1368-79. doi: 10.1016/j.freeradbiomed.2010.07.019 [Crossref] [ Google Scholar]
- Somparn N, Prawan A, Senggunprai L, Kukongviriyapan U, Jetsrisuparb A, Lee MH. Cellular adaptation mediated through Nrf2-induced glutamate cysteine ligase up-regulation against oxidative stress caused by iron overload in β-thalassemia/HbE patients. Free Radic Res 2019; 53(7):791-9. doi: 10.1080/10715762.2019.1632444 [Crossref] [ Google Scholar]
- Chen X, Wang Y, Zhu H, Hao H, Zhao X, Qin T. Comparative transcript profiling of gene expression of fresh and frozen-thawed bull sperm. Theriogenology 2015; 83(4):504-11. doi: 10.1016/j.theriogenology.2014.10.015 [Crossref] [ Google Scholar]